Nucleoprotein of influenza A virus negatively impacts antiapoptotic protein API5 to enhance E2F1-dependent apoptosis and virus replication
- PMID: 26673663
- PMCID: PMC4720893
- DOI: 10.1038/cddis.2015.360
Nucleoprotein of influenza A virus negatively impacts antiapoptotic protein API5 to enhance E2F1-dependent apoptosis and virus replication
Abstract
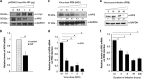
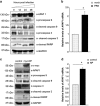
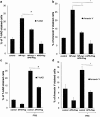
Apoptosis of host cells profoundly influences virus propagation and dissemination, events that are integral to influenza A virus (IAV) pathogenesis. The trigger for activation of apoptosis is regulated by an intricate interplay between cellular and viral proteins, with a strong bearing on IAV replication. Though the knowledge of viral proteins and mechanisms employed by IAV to induce apoptosis has advanced considerably of late, we know relatively little about the repertoire of host factors targeted by viral proteins. Thus, identification of cellular proteins that are hijacked by the virus will help us not only to understand the molecular underpinnings of IAV-induced apoptosis, but also to design future antiviral therapies. Here we show that the nucleoprotein (NP) of IAV directly interacts with and suppresses the expression of API5, a host antiapoptotic protein that antagonizes E2F1-dependent apoptosis. siRNA-mediated depletion of API5, in NP-overexpressed as well as IAV-infected cells, leads to upregulation of apoptotic protease activating factor 1 (APAF1), a downstream modulator of E2F1-mediated apoptosis, and cleavage of caspases 9 and 3, although a reciprocal pattern of these events was observed on ectopic overexpression of API5. In concordance with these observations, annexin V and 7AAD staining assays exhibit downregulation of early and late apoptosis in IAV-infected or NP-transfected cells on overexpression of API5. Most significantly, while overexpression of API5 decreases viral titers, cellular NP protein as well as mRNA levels in IAV-infected A549 cells, silencing of API5 expression causes a steep rise in the same parameters. From the data reported in this manuscript, we propose a proapoptotic role for NP in IAV pathogenesis, whereby it suppresses expression of antiapoptotic factor API5, thus potentiating the E2F1-dependent apoptotic pathway and ensuring viral replication.
Figures





Similar articles
-
Autophagy Promotes Replication of Influenza A Virus In Vitro.J Virol. 2019 Feb 5;93(4):e01984-18. doi: 10.1128/JVI.01984-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30541828 Free PMC article.
-
Bik Mediates Caspase-Dependent Cleavage of Viral Proteins to Promote Influenza A Virus Infection.Am J Respir Cell Mol Biol. 2016 May;54(5):664-73. doi: 10.1165/rcmb.2015-0133OC. Am J Respir Cell Mol Biol. 2016. PMID: 26437021 Free PMC article.
-
Inhibition of Ongoing Influenza A Virus Replication Reveals Different Mechanisms of RIG-I Activation.J Virol. 2019 Mar 5;93(6):e02066-18. doi: 10.1128/JVI.02066-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30602605 Free PMC article.
-
Relevance of signaling molecules for apoptosis induction on influenza A virus replication.Biochem Biophys Res Commun. 2013 Nov 22;441(3):531-7. doi: 10.1016/j.bbrc.2013.10.100. Epub 2013 Oct 28. Biochem Biophys Res Commun. 2013. PMID: 24177013 Free PMC article. Review.
-
Influenza virus nucleoprotein: structure, RNA binding, oligomerization and antiviral drug target.Future Microbiol. 2013 Dec;8(12):1537-45. doi: 10.2217/fmb.13.128. Future Microbiol. 2013. PMID: 24266354 Review.
Cited by
-
Betacyanins from red pitahaya (Hylocereus polyrhizus) exhibit antiviral response against influenza A virus.Heliyon. 2024 Jun 15;10(12):e33049. doi: 10.1016/j.heliyon.2024.e33049. eCollection 2024 Jun 30. Heliyon. 2024. PMID: 39021953 Free PMC article.
-
Identifying suitable reference genes for gene expression analysis in developing skeletal muscle in pigs.PeerJ. 2016 Dec 13;4:e2428. doi: 10.7717/peerj.2428. eCollection 2016. PeerJ. 2016. PMID: 27994956 Free PMC article.
-
Influenza A Virus: Host-Virus Relationships.Viruses. 2020 Aug 9;12(8):870. doi: 10.3390/v12080870. Viruses. 2020. PMID: 32784813 Free PMC article.
-
Regulation of mRNA export through API5 and nuclear FGF2 interaction.Nucleic Acids Res. 2020 Jun 19;48(11):6340-6352. doi: 10.1093/nar/gkaa335. Nucleic Acids Res. 2020. PMID: 32383752 Free PMC article.
-
Apoptosis Inhibitor 5: A Multifaceted Regulator of Cell Fate.Biomolecules. 2024 Jan 22;14(1):136. doi: 10.3390/biom14010136. Biomolecules. 2024. PMID: 38275765 Free PMC article. Review.
References
-
- 1O'Brien V. Viruses and apoptosis. J Gen Virol 1998; 79: 1833–1845. - PubMed
-
- 2Benedict CA, Norris PS, Ware CF. To kill or be killed: viral evasion of apoptosis. Nat Immunol 2002; 3: 1013–1018. - PubMed
-
- 3Hardwick JM. Viral interference with apoptosis. Semin Cell Dev Biol 1998; 9: 339–349. - PubMed
-
- 4Bantel H, Schulze-Osthoff K. Apoptosis in hepatitis C virus infection. Cell Death Differ 2003; 10(Suppl 1): S48–S58. - PubMed
-
- 5Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WS. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology 1996; 220: 152–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

