A versatile ex vivo technique for assaying tumor angiogenesis and microglia in the brain
- PMID: 26673818
- PMCID: PMC4811501
- DOI: 10.18632/oncotarget.6550
A versatile ex vivo technique for assaying tumor angiogenesis and microglia in the brain
Abstract
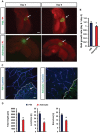
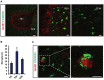
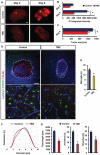
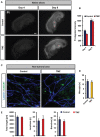
Primary brain tumors are hallmarked for their destructive activity on the microenvironment and vasculature. However, solely few experimental techniques exist to access the tumor microenvironment under anatomical intact conditions with remaining cellular and extracellular composition. Here, we detail an ex vivo vascular glioma impact method (VOGIM) to investigate the influence of gliomas and chemotherapeutics on the tumor microenvironment and angiogenesis under conditions that closely resemble the in vivo situation. We generated organotypic brain slice cultures from rats and transgenic mice and implanted glioma cells expressing fluorescent reporter proteins. In the VOGIM, tumor-induced vessels presented the whole range of vascular pathologies and tumor zones as found in human primary brain tumor specimens. In contrast, non-transformed cells such as primary astrocytes do not alter the vessel architecture. Vascular characteristics with vessel branching, junctions and vessel meshes are quantitatively assessable as well as the peritumoral zone. In particular, the VOGIM resembles the brain tumor microenvironment with alterations of neurons, microglia and cell survival. Hence, this method allows live cell monitoring of virtually any fluorescence-reporter expressing cell. We further analyzed the vasculature and microglia under the influence of tumor cells and chemotherapeutics such as Temozolamide (Temodal/Temcad®). Noteworthy, temozolomide normalized vasculare junctions and branches as well as microglial distribution in tumor-implanted brains. Moreover, VOGIM can be facilitated for implementing the 3Rs in experimentations. In summary, the VOGIM represents a versatile and robust technique which allows the assessment of the brain tumor microenvironment with parameters such as angiogenesis, neuronal cell death and microglial activity at the morphological and quantitative level.
Keywords: angiogenesis; ex vivo; glioblastoma; neuronal cell death; slice culture.
Conflict of interest statement
The authors declare no competing financial conflict of interests.
Figures
Similar articles
-
Sunitinib impedes brain tumor progression and reduces tumor-induced neurodegeneration in the microenvironment.Cancer Sci. 2015 Feb;106(2):160-70. doi: 10.1111/cas.12580. Epub 2015 Feb 15. Cancer Sci. 2015. PMID: 25458015 Free PMC article.
-
[Establishment and characterization of dual-color fluorescence nude mouse models of glioma].Zhonghua Zhong Liu Za Zhi. 2014 Feb;36(2):97-102. Zhonghua Zhong Liu Za Zhi. 2014. PMID: 24796456 Chinese.
-
Time lapse in vivo microscopy reveals distinct dynamics of microglia-tumor environment interactions-a new role for the tumor perivascular space as highway for trafficking microglia.Glia. 2016 Jul;64(7):1210-26. doi: 10.1002/glia.22994. Epub 2016 May 3. Glia. 2016. PMID: 27143298
-
Vascular microenvironment in gliomas.Cancer Treat Res. 2004;117:249-62. doi: 10.1007/978-1-4419-8871-3_15. Cancer Treat Res. 2004. PMID: 15015564 Review.
-
Angiogenesis and invasion in glioma.Brain Tumor Pathol. 2011 Feb;28(1):13-24. doi: 10.1007/s10014-010-0007-z. Epub 2011 Jan 8. Brain Tumor Pathol. 2011. PMID: 21221826 Review.
Cited by
-
Treatment with Cyclic AMP Activators Reduces Glioblastoma Growth and Invasion as Assessed by Two-Photon Microscopy.Cells. 2021 Mar 4;10(3):556. doi: 10.3390/cells10030556. Cells. 2021. PMID: 33806549 Free PMC article.
-
Glioblastoma research: US and international networking achievements.Oncotarget. 2017 Sep 26;8(70):115730-115735. doi: 10.18632/oncotarget.21270. eCollection 2017 Dec 29. Oncotarget. 2017. PMID: 29383196 Free PMC article. Review.
-
Combining organotypic tissue culture with light-sheet microscopy (OTCxLSFM) to study glioma invasion.EMBO Rep. 2023 Dec 6;24(12):e56964. doi: 10.15252/embr.202356964. Epub 2023 Nov 8. EMBO Rep. 2023. PMID: 37938214 Free PMC article.
-
Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system.Oncotarget. 2017 Dec 11;8(69):114393-114413. doi: 10.18632/oncotarget.23106. eCollection 2017 Dec 26. Oncotarget. 2017. PMID: 29371994 Free PMC article. Review.
-
Cytotoxic profiling of artesunic and betulinic acids and their synthetic hybrid compound on neurons and gliomas.Oncotarget. 2017 Jun 7;8(37):61457-61474. doi: 10.18632/oncotarget.18390. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977877 Free PMC article.
References
-
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. - PubMed
-
- Hick S, Fan Z, Buchfelder M, Eyupoglu IY, Savaskan N. Brain Tumor-Induced Angiogenesis: Approaches and Bioassays. In: Lichtor Terry., editor. Evolution of the Molecular Biology of Brain Tumors and the Therapeutic Implications. 2013. ISBN: 978-953-51-0989-1, InTech, Available from: http://wwwintechopencom/books/evolution-of-the-molecular-biology-of-brai.... - DOI
-
- Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229:180–91. - PubMed
-
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

