Degarelix therapy for prostate cancer in a real-world setting: experience from the German IQUO (Association for Uro-Oncological Quality Assurance) Firmagon® registry
- PMID: 26674089
- PMCID: PMC4681133
- DOI: 10.1186/s12894-015-0116-4
Degarelix therapy for prostate cancer in a real-world setting: experience from the German IQUO (Association for Uro-Oncological Quality Assurance) Firmagon® registry
Abstract
Background: We investigated the use of the gonadotropin-releasing hormone (GnRH) antagonist degarelix in everyday clinical practice using registry data from uro-oncology practices in Germany.
Methods: Data were analysed retrospectively from the IQUO (Association for uro-oncological quality assurance) patient registry. Data were prospectively collected from all consecutive PCa patients treated with degarelix (n = 1010) in 138 uro-oncology practices in Germany between May 2009 and December 2013.
Results: Median overall survival had not yet been reached in the all-patient group or in subgroups who had or had not received prior hormonal therapy (HT). Cox regression analysis showed that patients who had received prior HT (n = 542) had a 58 % increased mortality risk (hazard ratio 1.58, 95 % CI 1.20-2.09) versus patients who had not (n = 468) (p = 0.001). Also, in patients who had received prior luteinizing hormone-releasing hormone (LHRH) analogue therapy (LHRH agonists or GnRH antagonists), median time to PSA progression was shorter (209 weeks) than in those who had not received prior LHRH analogues (n = 555; median PSA progression-free survival not yet reached). Degarelix was generally well tolerated.
Conclusions: Degarelix was effective and well tolerated in everyday clinical practice, confirming observations from clinical studies. Patients who received prior HT appeared to have a significantly higher mortality risk.
Figures
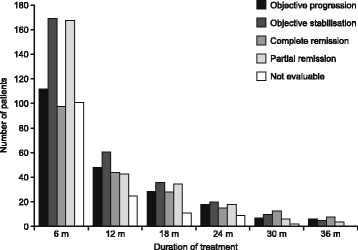
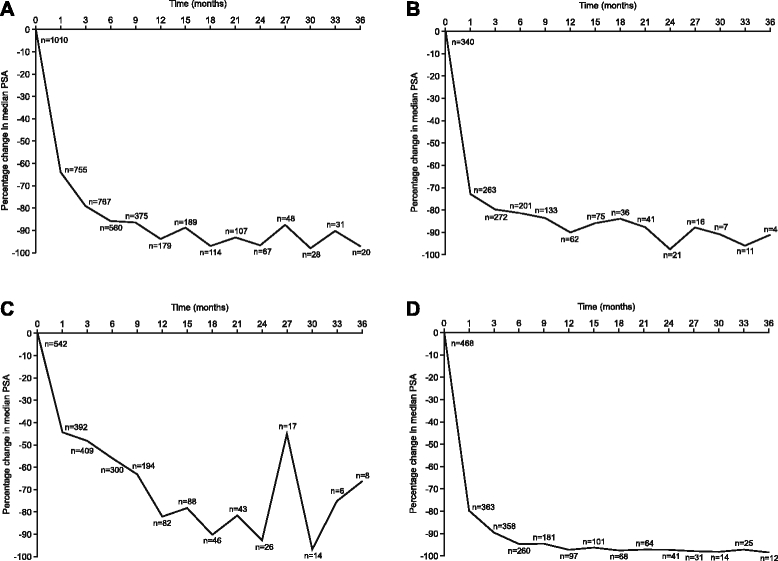
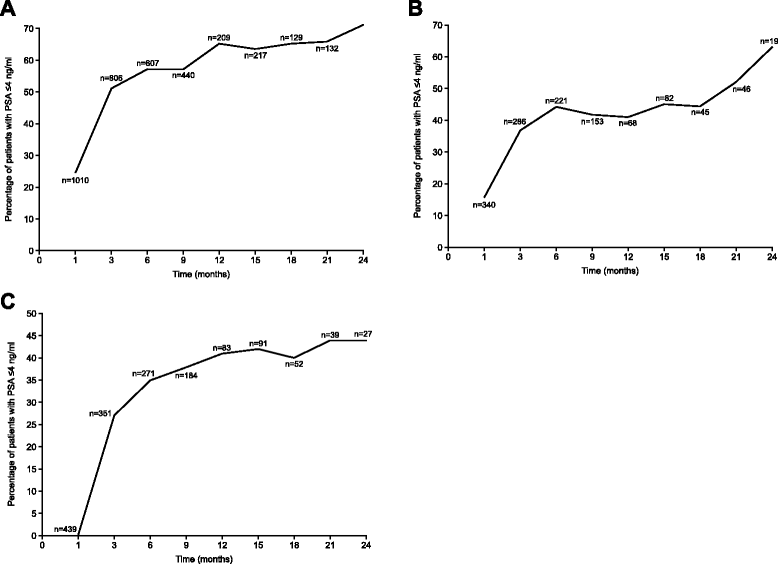
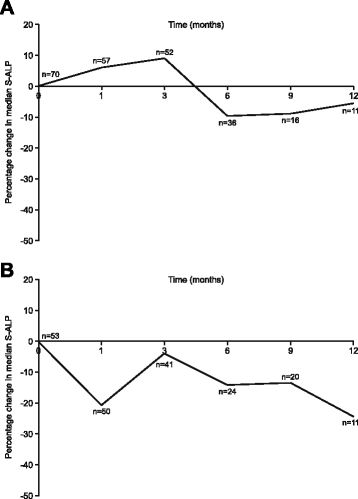
References
-
- Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int. 2008;102(11):1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x. - DOI - PubMed
-
- Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schroder F, Shore N, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol. 2011;186(3):889–897. doi: 10.1016/j.juro.2011.04.083. - DOI - PubMed
-
- Ozono S, Ueda T, Hoshi S, Yamaguchi A, Maeda H, Fukuyama Y, et al. The efficacy and safety of degarelix, a GnRH antagonist: a 12-month, multicentre, randomized, maintenance dose-finding phase II study in Japanese patients with prostate cancer. Jpn J Clin Oncol. 2012;42(6):477–484. doi: 10.1093/jjco/hys035. - DOI - PubMed
-
- Van Poppel H. LHRH agonists versus GnRH antagonists for the treatment of prostate cancer. Belgian J Med Oncol. 2010;4:18–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

