Mucopolysaccharidosis IIIB confers enhanced neonatal intracranial transduction by AAV8 but not by 5, 9 or rh10
- PMID: 26674264
- PMCID: PMC4777635
- DOI: 10.1038/gt.2015.111
Mucopolysaccharidosis IIIB confers enhanced neonatal intracranial transduction by AAV8 but not by 5, 9 or rh10
Abstract
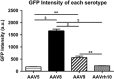
Sanfilippo syndrome type B (mucopolysaccharidosis IIIB, MPS IIIB) is a lysosomal storage disease resulting from deficiency of N-acetyl-glucosaminidase (NAGLU) activity. To determine the possible therapeutic utility of recombinant adeno-associated virus (rAAV) in early gene therapy-based interventions, we performed a comprehensive assessment of transduction and biodistribution profiles of four central nervous system (CNS) administered rAAV serotypes, -5, -8, -9 and -rh10. To simulate optimal earliest treatment of the disease, each rAAV serotype was injected into the CNS of neonatal MPS IIIB and control animals. We observed marked differences in biodistribution and transduction profiles between the serotypes and this differed in MPS IIIB compared with healthy control mice. Overall, in control mice, all serotypes performed comparably, although some differences were observed in certain focal areas. In MPS IIIB mice, AAV8 was more efficient than AAV5, -9 and -rh10 for gene delivery to most structures analyzed, including the cerebral cortex, hippocampus and thalamus. Noteworthy, the pattern of biodistribution within the CNS varied by serotype and genotype. Interestingly, AAV8 also produced the highest green fluorescent protein intensity levels compared with any other serotype and demonstrated improved transduction in NAGLU compared with control brains. Importantly, we also show leakage of AAV8, -9 and -rh10, but not AAV5, from CNS parenchyma to systemic organs. Overall, our data suggest that AAV8 represents the best therapeutic gene transfer vector for early intervention in MPS IIIB.
Figures




References
-
- Yogalingam G, Hopwood JJ. Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: diagnostic, clinical, and biological implications. Hum Mutat 2001; 18: 264–281. - PubMed
-
- Gilkes J, Patterson B, Heldermon C, Mucopolysaccharidosis III. Molecular genetics and genotype-phenotype correlations. OA Genet 2014; 2: 1.
-
- Fu H, DiRosario J, Kang L, Muenzer J, McCarty DM. Restoration of central nervous system α-N-acetylglucosaminidase activity and therapeutic benefits in mucopolysaccharidosis IIIB mice by a single intracisternal recombinant adeno-associated viral type 2 vector delivery. J Gene Med 2010; 12: 624–633. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

