Endocardial-to-mesenchymal transformation and mesenchymal cell colonization at the onset of human cardiac valve development
- PMID: 26674310
- PMCID: PMC4760315
- DOI: 10.1242/dev.133843
Endocardial-to-mesenchymal transformation and mesenchymal cell colonization at the onset of human cardiac valve development
Abstract
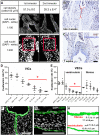
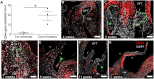
The elucidation of mechanisms in semilunar valve development might enable the development of new therapies for congenital heart disorders. Here, we found differences in proliferation-associated genes and genes repressed by VEGF between human semilunar valve leaflets from first and second trimester hearts. The proliferation of valve interstitial cells and ventricular valve endothelial cells (VECs) and cellular density declined from the first to the second trimester. Cytoplasmic expression of NFATC1 was detected in VECs (4 weeks) and, later, cells in the leaflet/annulus junction mesenchyme expressing inactive NFATC1 (5.5-9 weeks) were detected, indicative of endocardial-to-mesenchymal transformation (EndMT) in valvulogenesis. At this leaflet/annulus junction, CD44(+) cells clustered during elongation (11 weeks), extending toward the tip along the fibrosal layer in second trimester leaflets. Differing patterns of maturation in the fibrosa and ventricularis were detected via increased fibrosal periostin content, which tracked the presence of the CD44(+) cells in the second trimester. We revealed that spatiotemporal NFATC1 expression actively regulates EndMT during human valvulogenesis, as early as 4 weeks. Additionally, CD44(+) cells play a role in leaflet maturation toward the trilaminar structure, possibly via migration of VECs undergoing EndMT, which subsequently ascend from the leaflet/annulus junction.
Keywords: EndMT; Extracellular matrix; Heart; NFATc-1; Periostin; Semilunar valves.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



References
-
- Aikawa E., Whittaker P., Farber M., Mendelson K., Padera R. F., Aikawa M. and Schoen F. J. (2006). Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113, 1344-1352. 10.1161/CIRCULATIONAHA.105.591768 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

