Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct
- PMID: 26674313
- PMCID: PMC4674733
- DOI: 10.3748/wjg.v21.i46.13140
Endoscopic ultrasonography-guided drainage for patients with symptomatic obstruction and enlargement of the pancreatic duct
Abstract
Aim: To evaluate the use of translumenal pancreatography with placement of endoscopic ultrasonography (EUS)-guided drainage of the pancreatic duct.
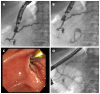
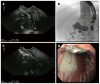
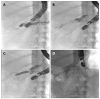
Methods: This study enrolled all consecutive patients between June 2002 and April 2014 who underwent EUS-guided pancreatography and subsequent placement of a drain and had symptomatic retention of fluid in the pancreatic duct after one or more previous unsuccessful attempts at endoscopic retrograde cannulation of the pancreatic duct. In all, 94 patients underwent 111 interventions with one of three different approaches: (1) EUS-endoscopic retrograde drainage with a rendezvous technique; (2) EUS-guided drainage of the pancreatic duct; and (3) EUS-guided, internal, antegrade drainage of the pancreatic duct.
Results: The mean duration of the interventions was 21 min (range, 15-69 min). Mean patient age was 54 years (range, 28-87 years); the M:F sex ratio was 60:34. The technical success rate was 100%, achieving puncture of the pancreatic duct including pancreatography in 94/94 patients. In patients requiring drainage, initial placement of a drain was successful in 47/83 patients (56.6%). Of these, 26 patients underwent transgastric/transbulbar positioning of a stent for retrograde drainage; plastic prostheses were used in 11 and metal stents in 12. A ring drain (antegrade internal drainage) was placed in three of these 26 patients because of anastomotic stenosis after a previous surgical intervention. The remaining 21 patients with successful drain placement had transpapillary drains using the rendezvous technique; the majority (n = 19) received plastic prostheses, and only two received metal stents (covered self-expanding metal stents). The median follow-up time in the 21 patients with transpapillary drainage was 28 mo (range, 1-79 mo), while that of the 26 patients with successful transgastric/transduodenal drainage was 9.5 mo (range, 1-82 mo). Clinical success, as indicated by reduced or absence of further pain after the EUS-guided intervention was achieved in 68/83 patients (81.9%), including several who improved without drainage, but with manipulation of the access route.
Conclusion: EUS-guided drainage of the pancreatic duct is a safe, feasible alternative to endoscopic retrograde drainage when the papilla cannot be reached endoscopically or catheterized.
Keywords: Clinical success; Endoscopic ultrasonography; Endoscopic ultrasonography-guided drainage of the pancreatic duct; Endoscopic ultrasonography-guided transmural pancreatography; Metal stent; Plastic prosthesis; Prospective, long-term, single-center study; Technical success.
Figures

Similar articles
-
Transgastric pancreatography and EUS-guided drainage of the pancreatic duct.J Hepatobiliary Pancreat Surg. 2007;14(4):377-82. doi: 10.1007/s00534-006-1139-8. Epub 2007 Jul 30. J Hepatobiliary Pancreat Surg. 2007. PMID: 17653636
-
Endoscopic ultrasonography-guided pancreatic duct drainage after failed endoscopic retrograde cholangiopancreatography in patients with malignant and benign pancreatic duct obstructions.Dig Endosc. 2013 May;25 Suppl 2:109-16. doi: 10.1111/den.12100. Dig Endosc. 2013. PMID: 23617660
-
Endoscopic ultrasonography-guided pancreatic duct access: techniques and literature review of pancreatography, transmural drainage and rendezvous techniques.Dig Endosc. 2013 May;25(3):241-52. doi: 10.1111/den.12048. Epub 2013 Mar 12. Dig Endosc. 2013. PMID: 23490022 Review.
-
EUS-guided biliary interventions for benign diseases and unsuccessful ERCP - a prospective unicenter feasibility study on a large consecutive patient cohort.Z Gastroenterol. 2021 Sep;59(9):933-943. doi: 10.1055/a-1540-7975. Epub 2021 Sep 10. Z Gastroenterol. 2021. PMID: 34507372 English.
-
Endoscopic ultrasound-guided pancreatic duct drainage: a comprehensive state of the art review.Expert Rev Gastroenterol Hepatol. 2024 Jul;18(7):351-365. doi: 10.1080/17474124.2024.2383631. Epub 2024 Jul 23. Expert Rev Gastroenterol Hepatol. 2024. PMID: 39041336 Review.
Cited by
-
Present status and perspectives of endosonography 2017 in gastroenterology.Korean J Intern Med. 2018 Jan;33(1):36-63. doi: 10.3904/kjim.2017.212. Epub 2017 Nov 23. Korean J Intern Med. 2018. PMID: 29161800 Free PMC article. Review.
-
Current paradigm of endoscopic ultrasound in biliary and pancreatic duct drainage: an update.Ann Gastroenterol. 2024 Jan-Feb;37(1):1-14. doi: 10.20524/aog.2023.0854. Epub 2024 Dec 23. Ann Gastroenterol. 2024. PMID: 38223246 Free PMC article. Review.
-
Therapeutic endoscopic ultrasound.Transl Gastroenterol Hepatol. 2022 Apr 25;7:20. doi: 10.21037/tgh-2020-12. eCollection 2022. Transl Gastroenterol Hepatol. 2022. PMID: 35548470 Free PMC article. Review.
-
Turkish Gastroenterology Association, Pancreas Study Group, Chronic Pancreatitis Committee Consensus Report.Turk J Gastroenterol. 2020 Nov;31(Supp1):S1-S41. doi: 10.5152/tjg.2020.220920. Turk J Gastroenterol. 2020. PMID: 33210608 Free PMC article. No abstract available.
-
EUS-guided pancreatic drainage: A steep learning curve.Endosc Ultrasound. 2020 May-Jun;9(3):175-179. doi: 10.4103/eus.eus_3_20. Endosc Ultrasound. 2020. PMID: 32584312 Free PMC article.
References
-
- Ebbehøj N, Borly L, Bülow J, Rasmussen SG, Madsen P, Matzen P, Owre A. Pancreatic tissue fluid pressure in chronic pancreatitis. Relation to pain, morphology, and function. Scand J Gastroenterol. 1990;25:1046–1051. - PubMed
-
- Widdison AL, Alvarez C, Karanjia ND, Reber HA. Experimental evidence of beneficial effects of ductal decompression in chronic pancreatitis. Endoscopy. 1991;23:151–154. - PubMed
-
- Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: A treatment algorithm. Best Pract Res Clin Gastroenterol. 2010;24:323–335. - PubMed
-
- Cotton PB. Endoscopic retrograde cholangiopancreatography: maximizing benefits and minimizing risks. Gastrointest Endosc Clin N Am. 2012;22:587–599. - PubMed
-
- Díte P, Ruzicka M, Zboril V, Novotný I. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy. 2003;35:553–558. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

