Phosphorylation of GSK3α/β correlates with activation of AKT and is prognostic for poor overall survival in acute myeloid leukemia patients
- PMID: 26674329
- PMCID: PMC4661707
- DOI: 10.1016/j.bbacli.2015.07.001
Phosphorylation of GSK3α/β correlates with activation of AKT and is prognostic for poor overall survival in acute myeloid leukemia patients
Abstract
Background: Acute myeloid leukemia (AML) patients with highly active AKT tend to do poorly. Cell cycle arrest and apoptosis are tightly regulated by AKT via phosphorylation of GSK3α and β isoforms which inactivates these kinases. In the current study we examine the prognostic role of AKT mediated GSK3 phosphorylation in AML.
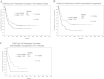
Methods: We analyzed GSK3α/β phosphorylation by reverse phase protein analysis (RPPA) in a cohort of 511 acute myeloid leukemia (AML) patients. Levels of phosphorylated GSK3 were correlated with patient characteristics including survival and with expression of other proteins important in AML cell survival.
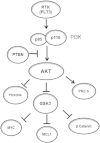
Results: High levels of p-GSK3α/β correlated with adverse overall survival and a lower incidence of complete remission duration in patients with intermediate cytogenetics, but not in those with unfavorable cytogenetics. Intermediate cytogenetic patients with FLT3 mutation also fared better respectively when p-GSK3α/β levels were lower. Phosphorylated GSK3α/β expression was compared and contrasted with that of 229 related cell cycle arrest and/or apoptosis proteins. Consistent with p-GSK3α/β as an indicator of AKT activation, RPPA revealed that p-GSK3α/β positively correlated with phosphorylation of AKT, BAD, and P70S6K, and negatively correlated with β-catenin and FOXO3A. PKCδ also positively correlated with p-GSK3α/β expression, suggesting crosstalk between the AKT and PKC signaling pathways in AML cells.
Conclusions: These findings suggest that AKT-mediated phosphorylation of GSK3α/β may be beneficial to AML cell survival, and hence detrimental to the overall survival of AML patients. Intrinsically, p-GSK3α/β may serve as an important adverse prognostic factor for a subset of AML patients.
Keywords: AKT; GSK3; Leukemia; PKC delta; RPPA; Signal transduction.
Figures







Similar articles
-
Role of MSC-derived galectin 3 in the AML microenvironment.Biochim Biophys Acta Mol Cell Res. 2018 Jul;1865(7):959-969. doi: 10.1016/j.bbamcr.2018.04.005. Epub 2018 Apr 12. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29655803 Free PMC article.
-
LGALS3 is connected to CD74 in a previously unknown protein network that is associated with poor survival in patients with AML.EBioMedicine. 2019 Jun;44:126-137. doi: 10.1016/j.ebiom.2019.05.025. Epub 2019 May 16. EBioMedicine. 2019. PMID: 31105032 Free PMC article.
-
Low expression of PP2A regulatory subunit B55α is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients.Leukemia. 2011 Nov;25(11):1711-7. doi: 10.1038/leu.2011.146. Epub 2011 Jun 10. Leukemia. 2011. PMID: 21660042 Free PMC article.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
GSK-3 as a novel prognostic indicator in leukemia.Adv Biol Regul. 2017 Aug;65:26-35. doi: 10.1016/j.jbior.2017.05.001. Epub 2017 May 8. Adv Biol Regul. 2017. PMID: 28499784 Review.
Cited by
-
Screening of autophagy genes as prognostic indicators for glioma patients.Am J Transl Res. 2020 Sep 15;12(9):5320-5331. eCollection 2020. Am J Transl Res. 2020. PMID: 33042422 Free PMC article.
-
Role of MSC-derived galectin 3 in the AML microenvironment.Biochim Biophys Acta Mol Cell Res. 2018 Jul;1865(7):959-969. doi: 10.1016/j.bbamcr.2018.04.005. Epub 2018 Apr 12. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29655803 Free PMC article.
-
Distinct protein signatures of acute myeloid leukemia bone marrow-derived stromal cells are prognostic for patient survival.Haematologica. 2018 May;103(5):810-821. doi: 10.3324/haematol.2017.172429. Epub 2018 Mar 15. Haematologica. 2018. PMID: 29545342 Free PMC article.
-
Shining a light on cell signaling in leukemia through proteomics: relevance for the clinic.Expert Rev Proteomics. 2018 Jul;15(7):613-622. doi: 10.1080/14789450.2018.1487781. Epub 2018 Jul 6. Expert Rev Proteomics. 2018. PMID: 29898608 Free PMC article. Review.
-
Notch signalling drives bone marrow stromal cell-mediated chemoresistance in acute myeloid leukemia.Oncotarget. 2016 Apr 19;7(16):21713-27. doi: 10.18632/oncotarget.7964. Oncotarget. 2016. PMID: 26967055 Free PMC article.
References
-
- Martelli A.M., Evangelisti C., Chiarini F., Grimaldi C., Manzoli L., McCubrey J.A. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin. Investig. Drugs. 2009;18:1333–1349. - PubMed
-
- Martelli A.M., Nyåkern M., Tabellini G., Bortul R., Tazzari P.L., Evangelisti C. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

