The Fungal Sexual Pheromone Sirenin Activates the Human CatSper Channel Complex
- PMID: 26674547
- PMCID: PMC4761407
- DOI: 10.1021/acschembio.5b00748
The Fungal Sexual Pheromone Sirenin Activates the Human CatSper Channel Complex
Abstract
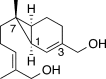
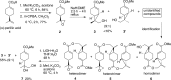
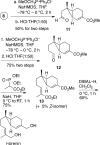
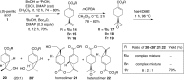
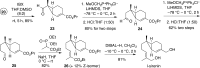
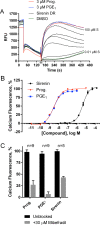
The basal fungus Allomyces macrogynus (A. macrogynus) produces motile male gametes displaying well-studied chemotaxis toward their female counterparts. This chemotaxis is driven by sirenin, a sexual pheromone released by the female gametes. The pheromone evokes a large calcium influx in the motile gametes, which could proceed through the cation channel of sperm (CatSper) complex. Herein, we report the total synthesis of sirenin in 10 steps and 8% overall yield and show that the synthetic pheromone activates the CatSper channel complex, indicated by a concentration-dependent increase in intracellular calcium in human sperm. Sirenin activation of the CatSper channel was confirmed using whole-cell patch clamp electrophysiology with human sperm. Based on this proficient synthetic route and confirmed activation of CatSper, analogues of sirenin can be designed as blockers of the CatSper channel that could provide male contraceptive agents.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Reyes A.; Goicoechea B.; Rosado A. (1978) Calcium ion requirement for rabbit spermatozoal capacitation and enhancement of fertilizing ability by ionophore A23187 and cyclic adenosine 3′:5′-monophosphate. Fertil. Steril. 29, 451–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN275201300017C/HD/NICHD NIH HHS/United States
- HHSN275201300017C/PHS HHS/United States
- R21HD081403/HD/NICHD NIH HHS/United States
- HHSN275201300017I/HD/NICHD NIH HHS/United States
- R01GM111802/GM/NIGMS NIH HHS/United States
- 1U01 HDO45857/PHS HHS/United States
- U01 HD045857/HD/NICHD NIH HHS/United States
- Howard Hughes Medical Institute/United States
- U01 HD076542/HD/NICHD NIH HHS/United States
- R01 GM111802/GM/NIGMS NIH HHS/United States
- R21 HD081403/HD/NICHD NIH HHS/United States
- 1U01HD076542/HD/NICHD NIH HHS/United States

