Estrogen Receptor α Regulates Dlx3-Mediated Osteoblast Differentiation
- PMID: 26674964
- PMCID: PMC4757804
- DOI: 10.14348/molcells.2016.2291
Estrogen Receptor α Regulates Dlx3-Mediated Osteoblast Differentiation
Abstract
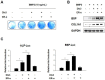
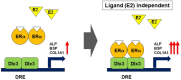
Estrogen receptor α (ER-α), which is involved in bone metabolism and breast cancer, has been shown to have transcriptional targets. Dlx3 is essential for the skeletal development and plays an important role in osteoblast differentiation. Various osteogenic stimulators and transcription factors can induce the protein expression of Dlx3. However, the regulatory function of ER-α in the Dlx3 mediated osteogenic process remains unknown. Therefore, we investigated the regulation of Dlx3 and found that ER-α is a positive regulator of Dlx3 transcription in BMP2-induced osteoblast differentiation. We also found that ER-α interacts with Dlx3 and increases its transcriptional activity and DNA binding affinity. Furthermore, we demonstrated that the regulation of Dlx3 activity by ER-α is independent of the ligand (estradiol) binding domain. These results indicate that Dlx3 is a novel target of ER-α, and that ER-α regulates the osteoblast differentiation through modulation of Dlx3 expression and/or interaction with Dlx3.
Keywords: Dlx3; estrogen receptor α; osteoblast differentiation.
Figures



Similar articles
-
Protein kinase a phosphorylates Dlx3 and regulates the function of Dlx3 during osteoblast differentiation.J Cell Biochem. 2014 Nov;115(11):2004-11. doi: 10.1002/jcb.24872. J Cell Biochem. 2014. PMID: 24924519
-
BMP2 commitment to the osteogenic lineage involves activation of Runx2 by DLX3 and a homeodomain transcriptional network.J Biol Chem. 2006 Dec 29;281(52):40515-26. doi: 10.1074/jbc.M604508200. Epub 2006 Oct 23. J Biol Chem. 2006. PMID: 17060321
-
Akt1 regulates phosphorylation and osteogenic activity of Dlx3.Biochem Biophys Res Commun. 2012 Sep 7;425(4):800-5. doi: 10.1016/j.bbrc.2012.07.155. Epub 2012 Aug 2. Biochem Biophys Res Commun. 2012. PMID: 22885182
-
Homeodomain transcription factors regulate BMP-2-induced osteoactivin transcription in osteoblasts.J Cell Physiol. 2012 Jan;227(1):390-9. doi: 10.1002/jcp.22791. J Cell Physiol. 2012. PMID: 21503878
-
Dlx3 transcriptional regulation of osteoblast differentiation: temporal recruitment of Msx2, Dlx3, and Dlx5 homeodomain proteins to chromatin of the osteocalcin gene.Mol Cell Biol. 2004 Oct;24(20):9248-61. doi: 10.1128/MCB.24.20.9248-9261.2004. Mol Cell Biol. 2004. PMID: 15456894 Free PMC article.
Cited by
-
Flightless-I governs cell fate by recruiting the SUMO isopeptidase SENP3 to distinct HOX genes.Epigenetics Chromatin. 2017 Mar 23;10:15. doi: 10.1186/s13072-017-0122-8. eCollection 2017. Epigenetics Chromatin. 2017. PMID: 28344658 Free PMC article.
-
Isopsoralen Enhanced Osteogenesis by Targeting AhR/ERα.Molecules. 2018 Oct 11;23(10):2600. doi: 10.3390/molecules23102600. Molecules. 2018. PMID: 30314280 Free PMC article.
-
Single-cell RNA sequencing analysis of the temporomandibular joint condyle in 3 and 4-month-old human embryos.Cell Biosci. 2023 Jul 19;13(1):130. doi: 10.1186/s13578-023-01069-5. Cell Biosci. 2023. PMID: 37468984 Free PMC article.
-
Effects of DLX3 on the osteogenic differentiation of induced pluripotent stem cell‑derived mesenchymal stem cells.Mol Med Rep. 2021 Apr;23(4):232. doi: 10.3892/mmr.2021.11871. Epub 2021 Jan 26. Mol Med Rep. 2021. PMID: 33655330 Free PMC article.
-
Breast-Specific Gamma Imaging with [99mTc]Tc-Sestamibi: An In Vivo Analysis for Early Identification of Breast Cancer Lesions Expressing Bone Biomarkers.J Clin Med. 2020 Mar 10;9(3):747. doi: 10.3390/jcm9030747. J Clin Med. 2020. PMID: 32164267 Free PMC article.
References
-
- Bjornstrom L., Sjoberg M. Mutations in the estrogen receptor DNA-binding domain discriminate between the classical mechanism of action and cross-talk with Stat5b and activating protein 1 (AP-1) J. Biol. Chem. 2002;277:48479–48483. - PubMed
-
- Chang W., Parra M., Centrella M., McCarthy T.L. Interactions between CCAAT enhancer binding protein delta and estrogen receptor alpha control insulin-like growth factor I (igf1) and estrogen receptor-dependent gene expression in osteoblasts. Gene. 2005;345:225–235. - PubMed
-
- Chen T.K., Smith L.M., Gebhardt D.K., Birrer M.J., Brown P.H. Activation and inhibition of the AP-1 complex in human breast cancer cells. Mol. Carcinog. 1996;15:215–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

