Angelica polymorpha Maxim Induces Apoptosis of Human SH-SY5Y Neuroblastoma Cells by Regulating an Intrinsic Caspase Pathway
- PMID: 26674967
- PMCID: PMC4757799
- DOI: 10.14348/molcells.2016.2232
Angelica polymorpha Maxim Induces Apoptosis of Human SH-SY5Y Neuroblastoma Cells by Regulating an Intrinsic Caspase Pathway
Abstract
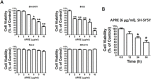
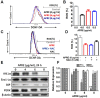
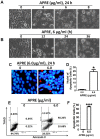
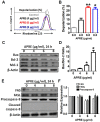
Angelica polymorpha Maxim root extract (APRE) is a popular herbal medicine used for treating stomachache, abdominal pain, stomach ulcers, and rheumatism; however the effect of APRE on cancer cells has not yet been explored. Here, we examined APRE cytotoxicity seen on target neuroblastoma cells (NB) using cell viability assays, DAPI visualization of fragmented DNA, and Western blotting analysis of candidate signaling pathways involved in proliferation and apoptosis. We demonstrated that APRE reduced cell viability in NB to a greater extent than in fibroblast cells. In addition, we found that APRE could inhibit the three classes of MAPK proteins and could also down-regulate the PI3K/AKT/GSK-3β activity all being relevant for proliferation and survival. APRE could also up-regulate Bax expression and down-regulate Bcl-2 and Mcl-1. With APRE treatment, depolarization of mitochondria membrane potential and activation of caspase-3 was demonstrated in the SH-SY5Y cells. We could not found increased activity of death receptor and caspase-8 as markers of the extrinsic apoptosis pathway for the APRE treated cells. In presence of a caspase-3 siRNA and a pan-caspase inhibitor, APRE could not reduce the viability of NB cells to a significant degree. So we predicted that with APRE, the intrinsic pathway was solely responsible for inducing apoptosis as we also showed that the non-caspase autophagy pathway or ER stress-ROS mediated pathways were not involved. These findings demonstrate that an intrinsic mitochondria-mediated apoptosis pathway mediates the apoptotic effects of APRE on SH-SY5Y cells, and that APRE shows promise as a novel agent for neuroblastoma therapy.
Keywords: Angelica polymorpha; Bax/Bcl-2 ratio; apoptosis; caspase; neuroblastoma.
Figures



Similar articles
-
Cytotoxic effect of gambogic acid on SH-SY5Y neuroblastoma cells is mediated by intrinsic caspase-dependent signaling pathway.Mol Cell Biochem. 2013 May;377(1-2):187-96. doi: 10.1007/s11010-013-1584-z. Epub 2013 Feb 13. Mol Cell Biochem. 2013. PMID: 23404459
-
Atrazine induces apoptosis of SH-SY5Y human neuroblastoma cells via the regulation of Bax/Bcl-2 ratio and caspase-3-dependent pathway.Pestic Biochem Physiol. 2015 Feb;118:90-8. doi: 10.1016/j.pestbp.2014.12.006. Epub 2014 Dec 12. Pestic Biochem Physiol. 2015. PMID: 25752436
-
Angelicin induces apoptosis through intrinsic caspase-dependent pathway in human SH-SY5Y neuroblastoma cells.Mol Cell Biochem. 2012 Oct;369(1-2):95-104. doi: 10.1007/s11010-012-1372-1. Epub 2012 Jul 6. Mol Cell Biochem. 2012. PMID: 22766766
-
Regulation of Intrinsic and Extrinsic Apoptotic Pathways in Osteosarcoma Cells Following Oleandrin Treatment.Int J Mol Sci. 2016 Nov 23;17(11):1950. doi: 10.3390/ijms17111950. Int J Mol Sci. 2016. PMID: 27886059 Free PMC article.
-
Traditional Chinese medicine targeting apoptotic mechanisms for esophageal cancer therapy.Acta Pharmacol Sin. 2016 Mar;37(3):295-302. doi: 10.1038/aps.2015.116. Epub 2015 Dec 28. Acta Pharmacol Sin. 2016. PMID: 26707140 Free PMC article. Review.
Cited by
-
Drug Target to Alleviate Mitochondrial Dysfunctions in Alzheimer's Disease: Recent Advances and Therapeutic Implications.Curr Neuropharmacol. 2024;22(12):1942-1959. doi: 10.2174/1570159X22666240426091311. Curr Neuropharmacol. 2024. PMID: 39234772 Free PMC article. Review.
-
Wound Healing-Promoting and Melanogenesis-Inhibiting Activities of Angelica polymorpha Maxim. Flower Absolute In Vitro and Its Chemical Composition.Molecules. 2021 Oct 13;26(20):6172. doi: 10.3390/molecules26206172. Molecules. 2021. PMID: 34684753 Free PMC article.
-
Molecular mechanisms of apoptosis in hepatocellular carcinoma cells induced by ethanol extracts of Solanum lyratum Thumb through the mitochondrial pathway.World J Gastroenterol. 2017 Feb 14;23(6):1010-1017. doi: 10.3748/wjg.v23.i6.1010. World J Gastroenterol. 2017. PMID: 28246474 Free PMC article.
-
Potential Therapeutic Role of Phytochemicals to Mitigate Mitochondrial Dysfunctions in Alzheimer's Disease.Antioxidants (Basel). 2020 Dec 28;10(1):23. doi: 10.3390/antiox10010023. Antioxidants (Basel). 2020. PMID: 33379372 Free PMC article. Review.
-
Targeting Autophagy with Natural Compounds in Cancer: A Renewed Perspective from Molecular Mechanisms to Targeted Therapy.Front Pharmacol. 2021 Aug 26;12:748149. doi: 10.3389/fphar.2021.748149. eCollection 2021. Front Pharmacol. 2021. PMID: 34512368 Free PMC article. Review.
References
-
- Bodur C., Basaga H. Bcl-2 inhibitors: emerging drugs in cancer therapy. Curr Med Chem. 2012;19:1804–1820. - PubMed
-
- Brodeur G.M. Neuroblastoma: biological insights into a clinical enigma. Nat. Rev. Cancer. 2003;3:203–216. - PubMed
-
- Castel V., Grau E., Noguera R., Martinez F. Molecular biology of neuroblastoma. Clin. Transl. Oncol. 2007;9:478–483. - PubMed
-
- Estaquier J., Francois V., Jean-Luc V., Bernard M. The mitochondrial pathways of apoptosis. Adv. Exp. Med. Biol. 2012;942:157–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

