The effect of androgens on ovarian follicle maturation: Dihydrotestosterone suppress FSH-stimulated granulosa cell proliferation by upregulating PPARγ-dependent PTEN expression
- PMID: 26674985
- PMCID: PMC4682139
- DOI: 10.1038/srep18319
The effect of androgens on ovarian follicle maturation: Dihydrotestosterone suppress FSH-stimulated granulosa cell proliferation by upregulating PPARγ-dependent PTEN expression
Abstract
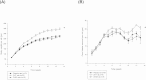
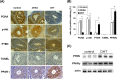
Intraovarian hyperandrogenism is one of the determining factors of follicular arrest in women with polycystic ovary syndrome (PCOS). Using androgenized rat models, we investigated the effects of androgens on metabolism, as well as on factors involved in follicular arrest and the reduced number of estrus cycles. The dihydrotestosterone (DHT)-treated rats had fewer estrus cycles, higher numbers of large arrested follicles and an increased in body weight gain compared with the dehydroepiandrostenedione (DHEA)- and placebo-treated rats. In cultured rat granulosa cells, DHT suppressed follicle stimulating hormone (FSH)-induced granulosa cell proliferation and increased the accumulation of cells in the G2/M phase. DHT decreased phosphorylated Akt (p-Akt) and cyclin D1 levels through increasing PTEN. DHT-promoted PTEN expression was regulated by peroxisome proliferator-activated receptor gamma (PPARγ) in granulosa cells. Meanwhile, in the large follicles of the DHT-treated rats, the expressions of PPARγ and PTEN were higher, but the expression of p-Akt and proliferating cell nuclear antigen (PCNA) were lower. Conclusively, DHT and DHEA produced differential effects on metabolism in prepubertal female rats like clinical manifestations of women with PCOS. DHT treatment may affect ovarian follicular maturation by altering granulosa cell proliferation through the regulation of enhancing PPARγ dependent PTEN/p-Akt expression in the granulosa cells.
Figures




References
-
- Franks S. Polycystic ovary syndrome. N Engl J Med 333, 853–61 (1995). - PubMed
-
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 23, 462–77 (2008). - PubMed
-
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Groups. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19, 41–7 (2004). - PubMed
-
- Azziz R. et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91, 4237–45 (2006). - PubMed
-
- Azziz R. et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91, 456–88 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

