Modeling Fatty Acid Transfer from Artery to Cardiomyocyte
- PMID: 26675003
- PMCID: PMC4682637
- DOI: 10.1371/journal.pcbi.1004666
Modeling Fatty Acid Transfer from Artery to Cardiomyocyte
Abstract
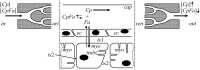
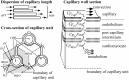
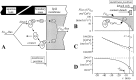
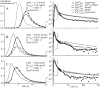
Despite the importance of oxidation of blood-borne long-chain fatty acids (Fa) in the cardiomyocytes for contractile energy of the heart, the mechanisms underlying the transfer of Fa from the coronary plasma to the cardiomyocyte is still incompletely understood. To obtain detailed insight into this transfer process, we designed a novel model of Fa transfer dynamics from coronary plasma through the endothelial cells and interstitium to the cardiomyocyte, applying standard physicochemical principles on diffusion and on the chemical equilibrium of Fa binding to carrier proteins Cp, like albumin in plasma and interstitium and Fatty Acid-Binding Proteins within endothelium and cardiomyocytes. Applying these principles, the present model strongly suggests that in the heart, binding and release of Fa to and from Cp in the aqueous border zones on both sides of the cell membranes form the major hindrance to Fa transfer. Although often considered, the membrane itself appears not to be a significant hindrance to diffusion of Fa. Proteins, residing in the cellular membrane, may facilitate transfer of Fa between Cp and membrane. The model is suited to simulate multiple tracer dilution experiments performed on isolated rabbit hearts administrating albumin and Fa as tracer substances into the coronary arterial perfusion line. Using parameter values on myocardial ultrastructure and physicochemical properties of Fa and Cp as reported in literature, simulated washout curves appear to be similar to the experimentally determined ones. We conclude therefore that the model is realistic and, hence, can be considered as a useful tool to better understand Fa transfer by evaluation of experimentally determined tracer washout curves.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Intra-cardiac transfer of fatty acids from capillary to cardiomyocyte.PLoS One. 2022 Jan 28;17(1):e0261288. doi: 10.1371/journal.pone.0261288. eCollection 2022. PLoS One. 2022. PMID: 35089937 Free PMC article.
-
Fatty acid transfer across the myocardial capillary wall.J Mol Cell Cardiol. 1993 Apr;25(4):355-66. doi: 10.1006/jmcc.1993.1042. J Mol Cell Cardiol. 1993. PMID: 8340929
-
Fatty acid transfer across the myocardial capillary wall: no evidence of a substantial role for cytoplasmic fatty acid-binding protein.J Mol Cell Cardiol. 1994 Dec;26(12):1635-47. doi: 10.1006/jmcc.1994.1183. J Mol Cell Cardiol. 1994. PMID: 7731058
-
Fatty acid binding to serum albumin: molecular simulation approaches.Biochim Biophys Acta. 2013 Dec;1830(12):5427-34. doi: 10.1016/j.bbagen.2013.03.032. Epub 2013 Apr 6. Biochim Biophys Acta. 2013. PMID: 23567799 Review.
-
What are the biochemical mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice?Cardiovasc Drugs Ther. 2008 Apr;22(2):83-9. doi: 10.1007/s10557-008-6088-9. Cardiovasc Drugs Ther. 2008. PMID: 18247111 Review.
Cited by
-
Mathematical Models Suggest Facilitated Fatty Acids Crossing of the Luminal Membrane in the Cardiac Muscle.J Membr Biol. 2017 Feb;250(1):103-114. doi: 10.1007/s00232-016-9941-y. Epub 2016 Dec 3. J Membr Biol. 2017. PMID: 27913823
-
Maximum Aerobic Function: Clinical Relevance, Physiological Underpinnings, and Practical Application.Front Physiol. 2020 Apr 2;11:296. doi: 10.3389/fphys.2020.00296. eCollection 2020. Front Physiol. 2020. PMID: 32300310 Free PMC article.
-
Intra-cardiac transfer of fatty acids from capillary to cardiomyocyte.PLoS One. 2022 Jan 28;17(1):e0261288. doi: 10.1371/journal.pone.0261288. eCollection 2022. PLoS One. 2022. PMID: 35089937 Free PMC article.
-
Fatty acid uptake in Trypanosoma brucei: Host resources and possible mechanisms.Front Cell Infect Microbiol. 2022 Nov 21;12:949409. doi: 10.3389/fcimb.2022.949409. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36478671 Free PMC article. Review.
-
The Physiological and Pathological Role of Acyl-CoA Oxidation.Int J Mol Sci. 2023 Oct 3;24(19):14857. doi: 10.3390/ijms241914857. Int J Mol Sci. 2023. PMID: 37834305 Free PMC article. Review.
References
-
- van der Vusse G.J., et al., Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev, 1992. 72(4): p. 881–940. - PubMed
-
- van der Vusse G.J., et al., Critical steps in cellular fatty acid uptake and utilization. Mol Cell Biochem, 2002. 239(1–2): p. 9–15. - PubMed
-
- Richieri G.V., Anel A., and Kleinfeld A.M., Interactions of long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry, 1993. 32(29): p. 7574–80. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

