In Vivo Assessment of Bone Regeneration in Alginate/Bone ECM Hydrogels with Incorporated Skeletal Stem Cells and Single Growth Factors
- PMID: 26675008
- PMCID: PMC4684226
- DOI: 10.1371/journal.pone.0145080
In Vivo Assessment of Bone Regeneration in Alginate/Bone ECM Hydrogels with Incorporated Skeletal Stem Cells and Single Growth Factors
Abstract
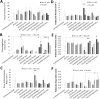
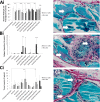
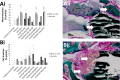
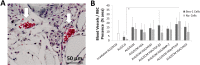
The current study has investigated the use of decellularised, demineralised bone extracellular matrix (ECM) hydrogel constructs for in vivo tissue mineralisation and bone formation. Stro-1-enriched human bone marrow stromal cells were incorporated together with select growth factors including VEGF, TGF-β3, BMP-2, PTHrP and VitD3, to augment bone formation, and mixed with alginate for structural support. Growth factors were delivered through fast (non-osteogenic factors) and slow (osteogenic factors) release PLGA microparticles. Constructs of 5 mm length were implanted in vivo for 28 days within mice. Dense tissue assessed by micro-CT correlated with histologically assessed mineralised bone formation in all constructs. Exogenous growth factor addition did not enhance bone formation further compared to alginate/bone ECM (ALG/ECM) hydrogels alone. UV irradiation reduced bone formation through degradation of intrinsic growth factors within the bone ECM component and possibly also ECM cross-linking. BMP-2 and VitD3 rescued osteogenic induction. ALG/ECM hydrogels appeared highly osteoinductive and delivery of angiogenic or chondrogenic growth factors led to altered bone formation. All constructs demonstrated extensive host tissue invasion and vascularisation aiding integration and implant longevity. The proposed hydrogel system functioned without the need for growth factor incorporation or an exogenous inducible cell source. Optimal growth factor concentrations and spatiotemporal release profiles require further assessment, as the bone ECM component may suffer batch variability between donor materials. In summary, ALG/ECM hydrogels provide a versatile biomaterial scaffold for utilisation within regenerative medicine which may be tailored, ultimately, to form the tissue of choice through incorporation of select growth factors.
Conflict of interest statement
Figures

Similar articles
-
Evaluation of skeletal tissue repair, part 2: enhancement of skeletal tissue repair through dual-growth-factor-releasing hydrogels within an ex vivo chick femur defect model.Acta Biomater. 2014 Oct;10(10):4197-205. doi: 10.1016/j.actbio.2014.05.025. Epub 2014 Jun 4. Acta Biomater. 2014. PMID: 24907660
-
Evaluation of skeletal tissue repair, part 1: assessment of novel growth-factor-releasing hydrogels in an ex vivo chick femur defect model.Acta Biomater. 2014 Oct;10(10):4186-96. doi: 10.1016/j.actbio.2014.06.011. Epub 2014 Jun 14. Acta Biomater. 2014. PMID: 24937137
-
Drug-Loadable Calcium Alginate Hydrogel System for Use in Oral Bone Tissue Repair.Int J Mol Sci. 2017 May 6;18(5):989. doi: 10.3390/ijms18050989. Int J Mol Sci. 2017. PMID: 28481253 Free PMC article.
-
In vivo analysis of hybrid hydrogels containing dual growth factor combinations, and skeletal stem cells under mechanical stimulation for bone repair.Mechanobiol Med. 2024 Aug 27;2(4):100096. doi: 10.1016/j.mbm.2024.100096. eCollection 2024 Dec. Mechanobiol Med. 2024. PMID: 40395225 Free PMC article.
-
Tissue engineered bone using select growth factors: A comprehensive review of animal studies and clinical translation studies in man.Eur Cell Mater. 2014 Oct 6;28:166-207; discussion 207-8. doi: 10.22203/ecm.v028a13. Eur Cell Mater. 2014. PMID: 25284140 Review.
Cited by
-
Human bone tissue-derived ECM hydrogels: Controlling physicochemical, biochemical, and biological properties through processing parameters.Bioact Mater. 2024 Sep 23;43:114-128. doi: 10.1016/j.bioactmat.2024.09.007. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39376928 Free PMC article.
-
Decellularized extracellular matrix materials for treatment of ischemic cardiomyopathy.Bioact Mater. 2023 Nov 29;33:460-482. doi: 10.1016/j.bioactmat.2023.10.015. eCollection 2024 Mar. Bioact Mater. 2023. PMID: 38076651 Free PMC article. Review.
-
Cellularizing hydrogel-based scaffolds to repair bone tissue: How to create a physiologically relevant micro-environment?J Tissue Eng. 2017 Jun 8;8:2041731417712073. doi: 10.1177/2041731417712073. eCollection 2017 Jan-Dec. J Tissue Eng. 2017. PMID: 28634532 Free PMC article.
-
An aorta ECM extracted hydrogel as a biomaterial in vascular tissue engineering application.Prog Biomater. 2022 Jun;11(2):207-217. doi: 10.1007/s40204-022-00186-7. Epub 2022 May 18. Prog Biomater. 2022. PMID: 35583849 Free PMC article.
-
Utility of extracellular matrix powders in tissue engineering.Organogenesis. 2018;14(4):172-186. doi: 10.1080/15476278.2018.1503771. Epub 2018 Sep 5. Organogenesis. 2018. PMID: 30183489 Free PMC article. Review.
References
-
- Marsh D. Concepts of fracture union, delayed union, and non-union. Clinical Oral Implants Research. 1998;(355 Supplement):S22–S30. - PubMed
-
- Ambikaipalan A, Wong JM, Khan WS. Preclinical and clinical studies on the use of stem cells for bone repair: a systematic review. Current Stem Cell Research and Therapy. 2013;8(3):210–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

