Long-term administration of high doses of transdermal buprenorphine in cancer patients with severe neuropathic pain
- PMID: 26675083
- PMCID: PMC4675634
- DOI: 10.2147/OTT.S91347
Long-term administration of high doses of transdermal buprenorphine in cancer patients with severe neuropathic pain
Abstract
Background: Buprenorphine is often administered by the transdermal route (transdermal buprenorphine [TB]) in cancer patients with severe neuropathic pain. However, high doses of TB of 140 µg/h are rarely used.
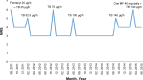
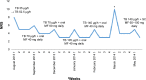
Patients and methods: Three cancer patients with severe neuropathic Numeric Rating Scale (NRS) pain scores of 8-10 who were successfully treated with high doses of TB up to 140 µg/h along with other opioids and adjuvant analgesics.
Results: TB was administered for a long period of follow-up (9 months to 4 years, including 34-261 days of treatment with the dose of 140 µg/h), which allowed achievement of satisfactory analgesia (NRS 3-5) and good treatment tolerance. In all three patients, TB dose was gradually titrated from 35 to 140 µg/h, and all patients used morphine at least for some time for breakthrough and background pain management along with adjuvant analgesics. Two patients continued the treatment with TB until the end of life, and one patient is still receiving the treatment.
Conclusion: TB at doses of up to 140 µg/h in cancer patients with severe neuropathic pain seems to be effective and safe in combination with other opioids and with adjuvant analgesics, and may significantly improve patients' quality of life. Clinical studies may explore higher than maximal 140 µg/h TB doses recommended by a manufacturer, and also in combination with other opioids and adjuvant analgesics.
Keywords: adverse effects; analgesia; cancer; neuropathic pain; transdermal buprenorphine; treatment.
Figures

References
-
- Sittl R. Transdermal buprenorphine in cancer pain and palliative care. Palliat Med. 2006;20(Suppl 1):s25–s30. - PubMed
-
- Sittl R, Griessinger N, Likar R. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, ran-domized, double-blind, placebo-controlled trial. Clin Ther. 2003;25:150–168. - PubMed
-
- Kusnik S, Likar R, Sittl R. Transdermal buprenorphine in chronic pain: indications and clinical experience. Expert Rev Clin Pharmacol. 2008;1:729–736. - PubMed
-
- Christoph T, Kögel B, Schiene K, Méen M, De Vry J, Friderichs E. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol. 2005;507:87–98. - PubMed
-
- Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. - PubMed
LinkOut - more resources
Full Text Sources

