Virulence-Dependent Alterations in the Kinetics of Immune Cells during Pulmonary Infection by Mycobacterium tuberculosis
- PMID: 26675186
- PMCID: PMC4682951
- DOI: 10.1371/journal.pone.0145234
Virulence-Dependent Alterations in the Kinetics of Immune Cells during Pulmonary Infection by Mycobacterium tuberculosis
Abstract
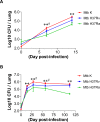
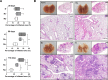
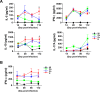
A better understanding of the kinetics of accumulated immune cells that are involved in pathophysiology during Mycobacterium tuberculosis (Mtb) infection may help to facilitate the development of vaccines and immunological interventions. However, the kinetics of innate and adaptive cells that are associated with pathogenesis during Mtb infection and their relationship to Mtb virulence are not clearly understood. In this study, we used a mouse model to compare the bacterial burden, inflammation and kinetics of immune cells during aerogenic infection in the lung between laboratory-adapted strains (Mtb H37Rv and H37Ra) and Mtb K strain, a hyper-virulent W-Beijing lineage strain. The Mtb K strain multiplied more than 10- and 3.54-fold more rapidly than H37Ra and H37Rv, respectively, during the early stage of infection (at 28 days post-infection) and resulted in exacerbated lung pathology at 56 to 112 days post-infection. Similar numbers of innate immune cells had infiltrated, regardless of the strain, by 14 days post-infection. High, time-dependent frequencies of F4/80-CD11c+CD11b-Siglec-H+PDCA-1+ plasmacytoid DCs and CD11c-CD11b+Gr-1int cells were observed in the lungs of mice that were infected with the Mtb K strain. Regarding adaptive immunity, Th1 and Th17 T cells that express T-bet and RORγt, respectively, significantly increased in the lungs that were infected with the laboratory-adapted strains, and the population of CD4+CD25+Foxp3+ regulatory T cells was remarkably increased at 112 days post-infection in the lungs of mice that were infected with the K strain. Collectively, our findings indicate that the highly virulent Mtb K strain may trigger the accumulation of pDCs and Gr1intCD11b+ cells with the concomitant down-regulation of the Th1 response and the maintenance of an up-regulated Th2 response without inducing a Th17 response during chronic infection. These results will help to determine which immune system components must be considered for the development of tuberculosis (TB) vaccines and immunological interventions.
Conflict of interest statement
Figures




References
-
- World Health Organization (2014) Global tuberculosis report 2014. World Health Organization, WHO/HTM/TB. 2014.08.
-
- Hunter RL, Actor JK, Hwang SA, Karev V, Jagannath C. Pathogenesis of post primary tuberculosis: immunity and hypersensitivity in the development of cavities. Ann Clin Lab Sci. 2014;44(4):365–87. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

