Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma
- PMID: 26675259
- PMCID: PMC4826192
- DOI: 10.18632/oncotarget.6547
Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma
Abstract
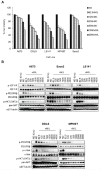
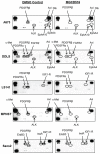
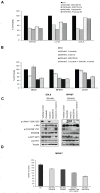
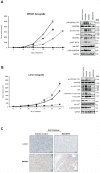
Sarcomas are rare but highly aggressive mesenchymal tumors with a median survival of 10-18 months for metastatic disease. Mutation and/or overexpression of many receptor tyrosine kinases (RTKs) including c-Met, PDGFR, c-Kit and IGF1-R drive defective signaling pathways in sarcomas. MGCD516 (Sitravatinib) is a novel small molecule inhibitor targeting multiple RTKs involved in driving sarcoma cell growth. In the present study, we evaluated the efficacy of MGCD516 both in vitro and in mouse xenograft models in vivo. MGCD516 treatment resulted in significant blockade of phosphorylation of potential driver RTKs and induced potent anti-proliferative effects in vitro. Furthermore, MGCD516 treatment of tumor xenografts in vivo resulted in significant suppression of tumor growth. Efficacy of MGCD516 was superior to imatinib and crizotinib, two other well-studied multi-kinase inhibitors with overlapping target specificities, both in vitro and in vivo. This is the first report describing MGCD516 as a potent multi-kinase inhibitor in different models of sarcoma, superior to imatinib and crizotinib. Results from this study showing blockade of multiple driver signaling pathways provides a rationale for further clinical development of MGCD516 for the treatment of patients with soft-tissue sarcoma.
Keywords: MGCD516; Sitravatinib; crizotinib; imatinib; tyrosine kinase.
Conflict of interest statement
The authors have no conflicts to disclose in relation to this work.
Figures


References
-
- Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. The New England journal of medicine. 2005;353:701–711. - PubMed
-
- Cormier JN, Pollock RE. Soft tissue sarcomas. CA. 2004;54:94–109. - PubMed
-
- Radaelli S, Stacchiotti S, Casali PG, Gronchi A. Emerging therapies for adult soft tissue sarcoma. Expert review of anticancer therapy. 2014;14:689–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

