Truncated HSPB1 causes axonal neuropathy and impairs tolerance to unfolded protein stress
- PMID: 26675522
- PMCID: PMC4661565
- DOI: 10.1016/j.bbacli.2015.03.002
Truncated HSPB1 causes axonal neuropathy and impairs tolerance to unfolded protein stress
Abstract
Background: HSPB1 belongs to the family of small heat shock proteins (sHSP) that have importance in protection against unfolded protein stress, in cancer cells for escaping drug toxicity stress and in neurons for suppression of protein aggregates. sHSPs have a conserved α-crystalline domain (ACD), flanked by variable N- and C-termini, whose functions are not fully understood. Dominant missense variants in HSPB1, locating mostly to the ACD, have been linked to inherited neuropathy.
Methods: Patients underwent detailed clinical and neurophysiologic characterization. Disease causing variants were identified by exome or gene panel sequencing. Primary patient fibroblasts were used to investigate the effects of the dominant defective HSPB1 proteins.
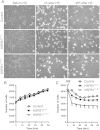
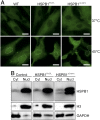
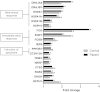
Results: Frameshift variant predicting ablation of the entire C-terminus p.(Met169Cfs2*) of HSPB1 and a missense variant p.(Arg127Leu) were identified in patients with dominantly inherited motor-predominant axonal Charcot-Marie-Tooth neuropathy. We show that the truncated protein is stable and binds wild type HSPB1. Both mutations impaired the heat stress tolerance of the fibroblasts. This effect was particularly pronounced for the cells with the truncating variant, independent of heat-induced nuclear translocation and induction of global transcriptional heat response. Furthermore, the truncated HSPB1 increased cellular sensitivity to protein misfolding.
Conclusion: Our results suggest that truncation of the non-conserved C-terminus impairs the function of HSPB1 in cellular stress response.
General significance: sHSPs have important roles in prevention of protein aggregates that induce toxicity. We showed that C-terminal part of HSPB1 is critical for tolerance of unfolded protein stress, and when lacking causes axonal neuropathy in patients.
Keywords: ACD, α-crystalline domain; CADD, combined annotation dependent depletion; CMT, Charcot–Marie–Tooth disease; Charcot–Marie–Tooth neuropathy; EMG, electromyography; ENMG, electroneuromyography; EVS, exome variant server; HSPB1; MUP, motor unit potential; Protein misfolding; QST, quantitative sensory testing; SISu, Sequencing Initiative Suomi; dHMN, distal hereditary motor neuropathy; heat shock protein; sHSP, small heat shock protein.
Figures



References
-
- Rossor A.M., Kalmar B., Greensmith L., Reilly M.M. The distal hereditary motor neuropathies. J. Neurol. Neurosurg. Psychiatry. 2012;83:6–14. - PubMed
-
- Rossor A.M., Polke J.M., Houlden H., Reilly M.M. Clinical implications of genetic advances in Charcot–Marie–Tooth disease. Nat. Rev. Neurol. 2013;9:562–571. - PubMed
-
- Mymrikov E.V., Seit-Nebi A.S., Gusev N.B. Large potentials of small heat shock proteins. Physiol. Rev. 2011;91:1123–1159. - PubMed
-
- Irobi J., Van Impe K., Seeman P., Jordanova A., Dierick I., Verpoorten N., Michalik A., De Vriendt E., Jacobs A., Van Gerwen V., Vennekens K., Mazanec R., Tournev I., Hilton-Jones D., Talbot K., Kremensky I., Van Den Bosch L., Robberecht W., Van Vandekerckhove J., Van Broeckhoven C., Gettemans J., De Jonghe P., Timmerman V. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004;36:597–601. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

