GluN2D N-Methyl-d-Aspartate Receptor Subunit Contribution to the Stimulation of Brain Activity and Gamma Oscillations by Ketamine: Implications for Schizophrenia
- PMID: 26675679
- PMCID: PMC4767398
- DOI: 10.1124/jpet.115.230391
GluN2D N-Methyl-d-Aspartate Receptor Subunit Contribution to the Stimulation of Brain Activity and Gamma Oscillations by Ketamine: Implications for Schizophrenia
Abstract
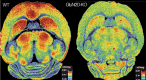
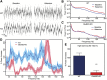
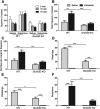
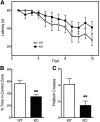
The dissociative anesthetic ketamine elicits symptoms of schizophrenia at subanesthetic doses by blocking N-methyl-d-aspartate receptors (NMDARs). This property led to a variety of studies resulting in the now well-supported theory that hypofunction of NMDARs is responsible for many of the symptoms of schizophrenia. However, the roles played by specific NMDAR subunits in different symptom components are unknown. To evaluate the potential contribution of GluN2D NMDAR subunits to antagonist-induced cortical activation and schizophrenia symptoms, we determined the ability of ketamine to alter regional brain activity and gamma frequency band neuronal oscillations in wild-type (WT) and GluN2D-knockout (GluN2D-KO) mice. In WT mice, ketamine (30 mg/kg, i.p.) significantly increased [(14)C]-2-deoxyglucose ([(14)C]-2DG) uptake in the medial prefrontal cortex (mPFC), entorhinal cortex and other brain regions, and decreased activity in the somatosensory cortex and inferior colliculus. In GluN2D-KO mice, however, ketamine did not significantly increase [(14)C]-2DG uptake in any brain region examined, yet still decreased [(14)C]-2DG uptake in the somatosensory cortex and inferior colliculus. Ketamine also increased locomotor activity in WT mice but not in GluN2D-KO mice. In electrocorticographic analysis, ketamine induced a 111% ± 16% increase in cortical gamma-band oscillatory power in WT mice, but only a 15% ± 12% increase in GluN2D-KO mice. Consistent with GluN2D involvement in schizophrenia-related neurologic changes, GluN2D-KO mice displayed impaired spatial memory acquisition and reduced parvalbumin (PV)-immunopositive staining compared with control mice. These results suggest a critical role of GluN2D-containing NMDARs in neuronal oscillations and ketamine's psychotomimetic, dissociative effects and hence suggests a critical role for GluN2D subunits in cognition and perception.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Abekawa T, Ito K, Nakagawa S, Koyama T. (2007) Prenatal exposure to an NMDA receptor antagonist, MK-801 reduces density of parvalbumin-immunoreactive GABAergic neurons in the medial prefrontal cortex and enhances phencyclidine-induced hyperlocomotion but not behavioral sensitization to methamphetamine in postpubertal rats. Psychopharmacology (Berl) 192:303–316. - PubMed
-
- Abel KM, Allin MP, Hemsley DR, Geyer MA. (2003) Low dose ketamine increases prepulse inhibition in healthy men. Neuropharmacology 44:729–737. - PubMed
-
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. (2008) Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet 40:827–834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

