Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
- PMID: 26676202
- PMCID: PMC6457737
- DOI: 10.1002/14651858.CD004706.pub5
Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer
Abstract
Background: This is the second update of the review first published in the Cochrane Database of Systematic Reviews in 2009, Issue 1. Epithelial ovarian cancer is diagnosed in over 200,000 women worldwide each year. Ten to 20% of women are diagnosed early, when there is still a good possibility of cure. The treatment of early-stage (stage I and IIa) disease involves surgery to remove the disease, often followed by chemotherapy (adjuvant chemotherapy). The largest clinical trials of adjuvant chemotherapy show an overall survival (OS) advantage with platinum-based chemotherapy; however the precise role and type of this treatment in subgroups of women with differing prognoses needs to be defined.
Objectives: To undertake a systematic review of the evidence for adjuvant chemotherapy in early-stage epithelial ovarian cancer to determine whether chemotherapy following surgery offers a survival advantage over the policy of observation following surgery (with chemotherapy reserved for treatment of disease recurrence); and to determine if clinical subgroups of women with differing prognoses, based on histological subtype or completeness of surgical staging, have more or less to gain from adjuvant chemotherapy.
Search methods: We performed an electronic search using the Cochrane Gynaecological Cancer Specialized Register, Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 3), MEDLINE (1948 to March week 5, 2015), and EMBASE (1980 to week 14, 2015). We developed the search strategy using free-text and medical subject headings (MeSH). We also searched registers of clinical trials and citation lists of included studies for potentially relevant studies.
Selection criteria: We included randomised clinical trials (RCTs) of women with early stage (I/IIa) epithelial ovarian cancer staged at laparotomy.
Data collection and analysis: Two review authors independently extracted data and assessed study quality of included RCTs. We resolved any disagreements by discussion with a third review author. We used random-effects methods for all meta-analyses, including subgroup analyses.
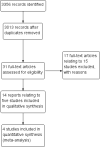
Main results: The original version of this Cochrane review included five RCTs involving 1277 women. In this 2015 update, no new studies met the inclusion criteria but we included an additional paper with mature data (10-year follow-up) relating to a previously included study (ICON1).We included four studies in the meta-analyses and considered them to be at a low risk of bias. Most study participants (> 95%) had stage I ovarian cancer. Meta-analysis of five-year data from three studies indicated that women who received adjuvant platinum-based chemotherapy had better overall survival (OS) than those who did not (Hazard ratio (HR) 0.71, 95% confidence interval (CI) 0.53 to 0.93; 1008 women; 3 studies; I² statistic = 0%; high quality evidence). Likewise, meta-analysis of five-year data from four studies indicated that women who received adjuvant chemotherapy had better progression-free survival (PFS) than those who did not (HR 0.67, 95% CI 0.53 to 0.84; 1170 women, 4 studies; I² statistic = 0%; high quality evidence). These findings were robust over time, with 10-year HR estimates of 0.72 (95% CI 0.57 to 0.92; 925 women, 2 studies) and 0.67 (95% CI 0.53 to 0.83; 925 women, 2 studies) for OS and PFS, respectively (high quality evidence). The risk of death at 10 years follow-up favoured the adjuvant chemotherapy arm (0.76, 95% CI 0.62 to 0.94; 923 women, 2 studies; I² statistic = 0%), as did the findings for risk of progression at 10 years (RR 0.72, 95% CI 0.60 to 0.87; 925 women, 2 studies; I² statistic = 0%). Low quality evidence suggested that women with high-risk disease may have the most to gain from adjuvant chemotherapy. However, subgroup analyses could neither confirm nor exclude survival benefits in lower risk disease or in optimally staged disease. We found insufficient data to compare adverse events and long term risks between chemotherapy and observation groups.
Authors' conclusions: High-quality evidence indicates that adjuvant platinum-based chemotherapy is effective in prolonging survival in women with early stage (FIGO stage I/IIa) epithelial ovarian cancer. It remains uncertain whether women with low- and intermediate-risk early stage disease will benefit as much from adjuvant chemotherapy as women with high-risk disease. Decisions to use adjuvant chemotherapy (AC) in these women should be mindful of this uncertainty, and the uncertainty regarding adverse events. Treatment of women with lower risk disease should be individualised to take into account individual factors.
Conflict of interest statement
BWR has no known conflicts of interest. TL has no known conflicts of interest. PH has no known conflicts of interest. HK has no known conflicts of interest.
Figures

























Update of
-
Adjuvant (post-surgery) chemotherapy for early stage epithelial ovarian cancer.Cochrane Database Syst Rev. 2012 Mar 14;3(3):CD004706. doi: 10.1002/14651858.CD004706.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Dec 17;(12):CD004706. doi: 10.1002/14651858.CD004706.pub5. PMID: 22419298 Free PMC article. Updated.
References
References to studies included in this review
ACTION 2003 {published data only}
-
- Timmers PJ, Zwinderman AH, Coens C, Vergote I, Trimbos JB. Understanding the problem of inadequately staging early ovarian cancer. European Journal of Cancer 2010;46(5):880‐4. - PubMed
-
- Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early‐stage ovarian carcinoma. Journal of the National Cancer Institute 2003;95(2):105‐12. - PubMed
-
- Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, et al. EORTC‐ACTION collaborators. European Organisation for Research and Treatment of Cancer‐Adjuvant ChemoTherapy in Ovarian Neoplasm. Impact of adjuvant chemotherapy and surgical staging in early‐stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer‐Adjuvant ChemoTherapy in Ovarian Neoplasm trial. Journal of the National Cancer Institute 2003;95(2):113‐25. - PubMed
-
- Vergote IB, Trimbos BJ, Guthrie D, Parmar M, Bolis G, Mangioni C, et al. Results of a randomized trial in 923 patients with high‐risk early ovarian cancer, comparing adjuvant chemotherapy with no further treatment following surgery [abstract]. Proceedings of the American Society of Clinical Oncology. 2001; Vol. 20 (Part 1):201a.
Bolis 1995 {published data only}
-
- Bolis G, Colombo N, Pecorelli S, Torri V, Marsoni S, Bonazzi C, et al. Adjuvant treatment for early epithelial ovarian cancer: results of two randomised clinical trials comparing cisplatin to no further treatment or chromic phosphate (32P). G.I.C.O.G.: Gruppo Interregionale Collaborativo in Ginecologia Oncologica. Annals of Oncology 1995;6(9):887‐93. - PubMed
ICON1 2003 {published data only}
-
- Colombo N, Guthrie D, Chiari S, Parmar M, Qian W, Swart AM, et al. International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early‐stage ovarian cancer. Journal of the National Cancer Institute 2003;95(2):125‐32. - PubMed
-
- Colombo N, Pecorelli S. What have we learned from ICON1 and ACTION?. International Journal of Gynecological Cancer 2003;13(Suppl 2):140‐3. - PubMed
-
- Colombo N, Trimbos JB, Guthrie D, Vergote I, Mangioni C, Vermorken J, et al. ACTION + ICON1: two parallel randomised phase III trials comparing adjuvant chemotherapy to no adjuvant chemotherapy following surgery in women with high risk early ovarian cancer [abstract]. European Journal of Cancer 2001;37(Suppl 6):276.
-
- International Collaborative Ovarian Neoplasm (ICON1) Collaborators. Erratum: "International collaborative ovarian neoplasm trial 1: A randomized trial of adjuvant chemotherapy in women with early‐stage ovarian cancer" (Journal of the National Cancer Institute (2003) vol. 95 (2) (125‐32)). Journal of the National Cancer Institute 2003;95(10):764. - PubMed
Tropé 2000 {published data only}
-
- Tropé C, Kaern J, Hogberg T, Abeler V, Hagen B, Kristensen G, et al. Randomized study on adjuvant chemotherapy in stage I high‐risk ovarian cancer with evaluation of DNA‐ploidy as prognostic instrument. Annals of Oncology 2000;11(3):281‐8. - PubMed
-
- Tropé C, Kaern J, Vergote I, Hagen B, Rosenberg P, Bertelsen K, et al. Randomized trial on adjuvant carboplatin versus no treatment in Stage I high risk ovarian cancer by the Nordic Ovarian Cancer Study Group (NOCOVA) [abstract]. Proceedings of the American Society of Clinical Oncology. 1997; Vol. 16:352a.
Young 1990 {published data only}
-
- Young RC, Walton LA, Ellenberg SS, Homesley HD, Wilbanks GD, Decker DG, et al. Adjuvant therapy in stage I and stage II epithelial ovarian cancer. Results of two prospective randomized trials. New England Journal of Medicine 1990;322(15):1021‐7. - PubMed
References to studies excluded from this review
Bapsy 2012 {published data only}
-
- Bapsy PP. Ovarian cancer. Journal of the Indian Medical Association 2012;110(12):894‐7. - PubMed
Bookman 2011 {published data only}
-
- Bookman MA. First line therapy: Have we made any improvement?. European Journal of Cancer 2011;47(Suppl 3):S93‐103. - PubMed
Burger 2012 {published data only}
-
- Burger RA. Advances in ovarian cancer disease control. Gynecologic Oncology 2012;124(1):5‐9. - PubMed
Cascales 2011 {published data only}
-
- Cascales PA, Gil J, Galindo PJ, Machado F, Frutos IM, Paricio PP. Heterogeneity in patients and methods. A problem for hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in ovarian carcinoma. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2011;158(2):361‐2. - PubMed
Chiara 1994 {published data only}
-
- Chiara S, Conte P, Franzone P, Orsatti M, Bruzzone M, Rubagotti A, et al. High‐risk early‐stage ovarian cancer. Randomized clinical trial comparing cisplatin plus cyclophosphamide versus whole abdominal radiotherapy. American Journal of Clinical Oncology 1994;17(1):72‐6. - PubMed
Cliby 2013 {published data only}
-
- Cliby W. Optimizing management tools for ovarian cancer improves survival. Gynecologic Oncology 2013;130(1):1‐2. - PubMed
Cui 2012 {published data only}
-
- Cui S, Ba M, Tang Y, Liu J, Wu Y, Wang B, et al. B ultrasound‐guided hyperthermic intraperitoneal perfusion chemotherapy for the treatment of malignant ascites. Oncology Reports 2012;28(4):1325‐31. - PubMed
Dembo 1979 {published data only}
-
- Dembo AJ, Bush RS, Beale FA, Bean HA, Pringle JF, Sturgeon J, et al. Ovarian carcinoma: improved survival following abdominopelvic irradiation in patients with a completed pelvic operation. American Journal of Obstetrics and Gynecology 1979;134(7):793‐800. - PubMed
Fujiwara 2012 {published data only}
Geurts 2011 {published data only}
-
- Geurts SM, Altena AM, Vegt F, Tjan‐Heijnen VC, Massuger LF, Dijck JA, et al. No supportive evidence for clinical benefit of routine follow‐up in ovarian cancer: a Dutch multicenter study. International Journal of Gynecological Cancer 2011;21(4):647‐53. - PubMed
Grönroos 1984 {published data only}
-
- Grönroos M, Nieminen U, Kauppila A, Kauppila O, Saksela E, Väyrynen M. A prospective, randomized, national trial for treatment of ovarian cancer: the role of chemotherapy and external irradiation. European Journal of Obstetrics, Gynaecology, and Reproductive Biology 1984;17(1):33‐42. - PubMed
Hreshchyshyn 1980 {published data only}
-
- Hreshchyshyn MM, Park RC, Blessing JA, Norris HJ, Levy D, Lagasse LD, et al. The role of adjuvant therapy in Stage I ovarian cancer. American Journal of Obstetrics and Gynecology 1980;138(2):139‐45. - PubMed
Klaassen 1988 {published data only}
-
- Dent SF, Klaassen D, Pater JL, Zee B, Whitehead M. Second primary malignancies following the treatment of early stage ovarian cancer: update of a study by the National Cancer Institute of Canada‐‐Clinical Trials Group (NCIC‐CTG). Annals of Oncology 2000;11(1):65‐8. - PubMed
-
- Klaassen D, Shelley W, Starreveld A, Kirk M, Boyes D, Gerulath A, et al. Early stage ovarian cancer: a randomized clinical trial comparing whole abdominal radiotherapy, melphalan, and intraperitoneal chromic phosphate: a National Cancer Institute of Canada Clinical Trials Group report. Journal of Clinical Oncology 1988;6(8):1254‐63. - PubMed
Kojs 2001 {published data only}
-
- Kojs Z, Glinski B, Reinfuss M, Pudelek J, Urbanski K, Kowalska T, et al. Results of a randomized prospective trial comparing postoperative abdominopelvic radiotherapy with postoperative chemotherapy in early ovarian cancer [Résultats d’un essai prospectif randomisé comparant une radiothérapie abdominopelvienne postopératoire et une chimiothérapie postopératoire dans les cancers de l’ovaire précoces]. Cancer/Radiothérapie 2001;5(1):5‐11. - PubMed
Maggioni 2006 {published data only}
Mannel 2011 {published data only}
-
- Mannel RS, Brady MF, Kohn EC, Hanjani P, Hiura M, Lee R, et al. A randomized phase III trial of IV carboplatin and paclitaxel × 3 courses followed by observation versus weekly maintenance low‐dose paclitaxel in patients with early‐stage ovarian carcinoma: a Gynecologic Oncology Group Study. Gynecological Oncology 2011;122(1):89‐94. - PMC - PubMed
Sell 1990 {published data only}
-
- Sell A, Bertelsen K, Andersen JE, Strøyer I, Panduro J. Randomized study of whole‐abdomen irradiation versus pelvic irradiation plus cyclophosphamide in treatment of early ovarian cancer. Gynecologic Oncology 1990;37(3):367‐73. - PubMed
Sevelda 1987 {published data only}
-
- Sevelda P, Gitsch E, Dittrich C, Haider F, Czerwenka K, Schemper M, et al. Therapeutic and prognostic results of a prospective multicenter ovarian cancer study of FIGO stages I and II [Therapeutische und prognostische Ergebnisse einer prospektiven multizentrischen Ovarialkarzinomstudie der FIGO‐Stadien I und II]. Geburtshilfe und Frauenheilkunde 1987;47(3):179‐85. - PubMed
Sigurdsson 1982 {published data only}
-
- Sigurdsson K, Johnsson JE, Tropé C. Carcinoma of the ovary, stages I and II. A prospective randomized study of the effects of postoperative chemotherapy and radiotherapy. Annales Chirurgiae et Gynaecologiae 1982;71(6):321‐9. - PubMed
Smith 1975 {published data only}
-
- Smith JP, Rutledge FN, Delclos L. Results of chemotherapy as an adjunct to surgery in patients with localized ovarian cancer. Seminars in Oncology 1975;2(3):277‐81. - PubMed
Vergote 1992 {published data only}
-
- Vergote IB, Vergote‐De Vos LN, Abeler VM, Aas M, Lindegaard MW, Kjørstad KE, et al. Randomized trial comparing cisplatin with radioactive phosphorus or whole‐abdomen irradiation as adjuvant treatment of ovarian cancer. Cancer 1992;69(3):741‐9. - PubMed
von Greunigen 2012 {published data only}
Young 2000 {published data only}
-
- Bell J, Brady MF, Young RC, LageJ, Walker JL, Look KY, et al. Randomised phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;102(3):432‐9. - PubMed
-
- Young RC. Three cycles versus six cycles of adjuvant paclitaxel (Taxol)/carboplatin in early stage ovarian cancer. Seminars in Oncology 2000;27(3 Suppl 7):8‐10. - PubMed
Young 2003 {published data only}
-
- Young RC, Brady MF, Nieberg RK, Long HJ, Mayer AR, Lentz SS, et al. Adjuvant treatment for early ovarian cancer: a randomized phase III trial of intraperitoneal 32P or intravenous cyclophosphamide and cisplatin‐‐a gynecologic oncology group study. Journal of Clinical Oncology 2003;21(23):4350‐5. - PubMed
Additional references
Altman 1995
AOCTG 1999
Bell 2006
-
- Bell J, Brady MF, Young RC, Lage J, Walker JL, Look KY, et al. Randomised phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;102(3):432‐9. - PubMed
Calvert 1989
-
- Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, et al. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. Journal of Clinical Oncology 1989;11(7):1748‐56. - PubMed
Collinson 2014
Colombo 2003
-
- Colombo N, Guthrie D, Chiari S, Parmar M, Qian W, Swart AM, et al. International Collaborative Ovarian Neoplasm trial 1: a randomized trial of adjuvant chemotherapy in women with early‐stage ovarian cancer. Journal of the National Cancer Institute 2003;95(2):125‐32. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Elit 2004
-
- Elit L, Chambers A, Fyles A, Covens A, Carey M, Fung MF. Systematic review of adjuvant care for women with stage I ovarian carcinoma. Cancer 2004;101(9):1926‐35. - PubMed
GLOBOCAN 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr (accessed dd Month yyyy).
GOG111 1996
-
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. New England Journal of Medicine 1996;334(1):1‐6. - PubMed
Green 2003
-
- Green JA. Early ovarian cancer‐‐time for a rethink on stage. Gynecologic Oncology 2003;90(2):235‐7. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICON2 1998
-
- ICON collaborators. ICON2: randomised trial of single agent carboplatin against three‐drug combination of CAP (cyclophosphamide, doxorubicin, and cisplatin) in women with ovarian cancer. ICON Collaborators. International Collaborative Ovarian Neoplasm Study. Lancet 1998;352(9140):1571‐6. - PubMed
Jemal 2008
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer Statistics, 2008. CA: a Cancer Journal for Clinicians 2008;58(2):71‐96. - PubMed
Mayer 1992
-
- Mayer AR, Chambers SK, Graves E, Holm C, Tseng PC, Nelson BE, et al. Ovarian cancer staging: does it require a gynecologic oncologist?. Gynecologic Oncology 1992;47(2):223‐7. - PubMed
Morice 2001
-
- Morice P, Wicart‐Poque F, Rey A, El‐Hassan J, Pautier P, Lhommé C, et al. Results of conservative treatment in epithelial ovarian carcinoma. Cancer 2001;92(9):2412‐8. - PubMed
NCI CTCAE v3.0 2006
-
- National Cancer Institute. National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 (NCI CTCAE v3.0). August 9 2006. http://www.eortc.be/services/doc/ctc/ctcaev3.pdf (accessed August 2006).
NICE 2011
-
- National Collaborating Centre for Cancer. Ovarian cancer: recognition and initial management: NICE clinical guideline 122. April 2011. https://www.nice.org.uk/guidance/cg122/resources/ovarian‐cancer‐recognit... (accessed April 2011). - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Peto 1982
-
- Peto R. Statistical aspects of cancer trials. In: Halnan KE editor(s). Treatment of Cancer. London: Chapman and Hall, 1982.
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sant 2003
-
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14(Suppl 5):v61‐118. [Supplement 5] - PubMed
Schilder 2002
-
- Schilder JM, Thompson AM, DePriest PD, Ueland FR, Cibull ML, Kryscio RJ, et al. Outcome of reproductive age women with stage IA or IC invasive epithelial ovarian cancer treated with fertility‐sparing therapy. Gynecologic Oncology 2002;87(1):1‐7. - PubMed
Shepherd 1989
-
- Shepherd JH. Revised FIGO staging for gynaecological cancer. British Journal of Obstetrics and Gynaecology 1989;96(8):889‐92. - PubMed
Swart 2007
-
- Swart AC, on behalf of ICON collaborators. Long‐term follow‐up of women enrolled in a randomized trial of adjuvant chemotherapy in early stage ovarian cancer (ICON1). Journal of Clinical Oncology. Chicago: ASCO Annual Meeting Proceedings, 2007; Vol. 25, No. 18S (June 20 Supplement):5509.
Thigpen 2011
-
- Thigpen T, duBois A, McAlpine J, DiSaia P, Fujiwara K, Hoskins W, et al. the Gynecologic Cancer InterGroup. First line therapy in ovarian cancer trials. International Journal of Gynecological Cancer 2011;21(4):756‐62. - PubMed
Timmers 2010
-
- Timmers PJ, Zwinderman AH, Coens C, Vergote I, Trimbos JB. Understanding the problem of inadequately staging early ovarian cancer. European Journal of Cancer 2010;46(5):880‐4. - PubMed
Trimbos 2003
-
- Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, et al. EORTC‐ACTION collaborators. European Organisation for Research and Treatment of Cancer‐Adjuvant ChemoTherapy in Ovarian Neoplasm. Impact of adjuvant chemotherapy and surgical staging in early‐stage ovarian carcinoma. Journal of the National Cancer Institute 2003;95(2):113‐25. - PubMed
Trimbos 2010
Tropé 2007
-
- Tropé C, Kaern J. Adjuvant chemotherapy for early‐stage ovarian cancer: review of the literature. Journal of Clinical Oncology 2007;25(20):2909‐20. - PubMed
Vergote 2001
-
- Vergote I, Brabanter J, Fyles A, Bertelsen K, Einhorn N, Selvelda P, et al. Prognostic importance of degree of differentiation and cyst rupture in stage 1 invasive epithelial carcinoma. Lancet 2001;357(9251):176‐82. - PubMed
Winter‐Roach 2003
-
- Winter‐Roach B, Hooper L, Kitchener H. Systematic review of adjuvant therapy for early stage (epithelial) ovarian cancer. International Journal of Gynecological Cancer 2003;13(4):395‐404. - PubMed
Zanetta 1998
-
- Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Torri V, et al. The accuracy of staging: an important prognostic determinator in stage I ovarian carcinoma. A multivariate analysis. Annals of Oncology 1998;9(10):1097‐101. - PubMed
References to other published versions of this review
Winter‐Roach 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

