Hypoxemia, oxygen content, and the regulation of cerebral blood flow
- PMID: 26676248
- PMCID: PMC4796739
- DOI: 10.1152/ajpregu.00270.2015
Hypoxemia, oxygen content, and the regulation of cerebral blood flow
Abstract
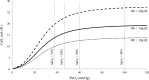
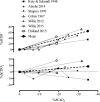
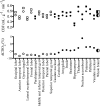
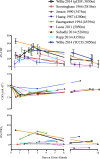
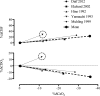
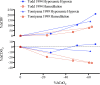
This review highlights the influence of oxygen (O2) availability on cerebral blood flow (CBF). Evidence for reductions in O2 content (CaO2 ) rather than arterial O2 tension (PaO2 ) as the chief regulator of cerebral vasodilation, with deoxyhemoglobin as the primary O2 sensor and upstream response effector, is discussed. We review in vitro and in vivo data to summarize the molecular mechanisms underpinning CBF responses during changes in CaO2 . We surmise that 1) during hypoxemic hypoxia in healthy humans (e.g., conditions of acute and chronic exposure to normobaric and hypobaric hypoxia), elevations in CBF compensate for reductions in CaO2 and thus maintain cerebral O2 delivery; 2) evidence from studies implementing iso- and hypervolumic hemodilution, anemia, and polycythemia indicate that CaO2 has an independent influence on CBF; however, the increase in CBF does not fully compensate for the lower CaO2 during hemodilution, and delivery is reduced; and 3) the mechanisms underpinning CBF regulation during changes in O2 content are multifactorial, involving deoxyhemoglobin-mediated release of nitric oxide metabolites and ATP, deoxyhemoglobin nitrite reductase activity, and the downstream interplay of several vasoactive factors including adenosine and epoxyeicosatrienoic acids. The emerging picture supports the role of deoxyhemoglobin (associated with changes in CaO2 ) as the primary biological regulator of CBF. The mechanisms for vasodilation therefore appear more robust during hypoxemic hypoxia than during changes in CaO2 via hemodilution. Clinical implications (e.g., disorders associated with anemia and polycythemia) and future study directions are considered.
Keywords: adenosine triphosphate; cerebral blood flow; cerebral oxygen delivery; hypoxia; nitric oxide.
Copyright © 2016 the American Physiological Society.
Figures



References
-
- Adelstein R, Conti M. Phosphorylation of smooth muscle myosin catalytic subunit of adenosine 3′,5′-monophosphate-dependent protein kinase. J Biol Chem 253: 8347–8350, 1978. - PubMed
-
- Ainslie PN, Ogoh S. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Exp Physiol 95: 251–262, 2010. - PubMed
-
- Ainslie PN, Poulin MJ. Ventilatory, cerebrovascular, and cardiovascular interactions in acute hypoxia: regulation by carbon dioxide. J Appl Physiol 97: 149–159, 2004. - PubMed
-
- Ainslie PN, Shaw AD, Smith KJ, Willie CK, Ikeda K, Graham J, Macleod DB. Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci (Lond) 126: 661–670, 2014. - PubMed
-
- Ainslie PN, Subudhi AW. Cerebral blood flow at high altitude. High Alt Med Biol 15: 133–140, 2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

