Ester Cross-Link Profiling of the Cutin Polymer of Wild-Type and Cutin Synthase Tomato Mutants Highlights Different Mechanisms of Polymerization
- PMID: 26676255
- PMCID: PMC4734573
- DOI: 10.1104/pp.15.01620
Ester Cross-Link Profiling of the Cutin Polymer of Wild-Type and Cutin Synthase Tomato Mutants Highlights Different Mechanisms of Polymerization
Abstract
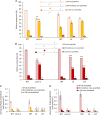
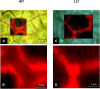
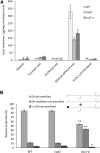
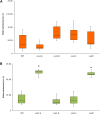
Cuticle function is closely related to the structure of the cutin polymer. However, the structure and formation of this hydrophobic polyester of glycerol and hydroxy/epoxy fatty acids has not been fully resolved. An apoplastic GDSL-lipase known as CUTIN SYNTHASE1 (CUS1) is required for cutin deposition in tomato (Solanum lycopersicum) fruit exocarp. In vitro, CUS1 catalyzes the self-transesterification of 2-monoacylglycerol of 9(10),16-dihydroxyhexadecanoic acid, the major tomato cutin monomer. This reaction releases glycerol and leads to the formation of oligomers with the secondary hydroxyl group remaining nonesterified. To check this mechanism in planta, a benzyl etherification of nonesterified hydroxyl groups of glycerol and hydroxy fatty acids was performed within cutin. Remarkably, in addition to a significant decrease in cutin deposition, mid-chain hydroxyl esterification of the dihydroxyhexadecanoic acid was affected in tomato RNA interference and ethyl methanesulfonate-cus1 mutants. Furthermore, in these mutants, the esterification of both sn-1,3 and sn-2 positions of glycerol was impacted, and their cutin contained a higher molar glycerol-to-dihydroxyhexadecanoic acid ratio. Therefore, in planta, CUS1 can catalyze the esterification of both primary and secondary alcohol groups of cutin monomers, and another enzymatic or nonenzymatic mechanism of polymerization may coexist with CUS1-catalyzed polymerization. This mechanism is poorly efficient with secondary alcohol groups and produces polyesters with lower molecular size. Confocal Raman imaging of benzyl etherified cutins showed that the polymerization is heterogenous at the fruit surface. Finally, by comparing tomato mutants either affected or not in cutin polymerization, we concluded that the level of cutin cross-linking had no significant impact on water permeance.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Baker EA, Bukovac MJ, Hunt GM (1982) Composition of tomato fruit cuticle as related to fruit growth and development. In Cutler DF, Alvin KL, Price CE, eds, The Plant Cuticle. Academic Press, London, pp 33–44
-
- Bargel H, Neinhuis C (2005) Tomato (Lycopersicon esculentum Mill.) fruit growth and ripening as related to the biomechanical properties of fruit skin and isolated cuticle. J Exp Bot 56: 1049–1060 - PubMed
-
- Beisson F, Li-Beisson Y, Pollard M (2012) Solving the puzzles of cutin and suberin polymer biosynthesis. Curr Opin Plant Biol 15: 329–337 - PubMed
-
- Benítez JJ, Heredia-Guerrero JA, Guzmán-Puyol S, Domínguez E, Heredia A (2015) Polyester films obtained by noncatalyzed melt-condensation polymerization of aleuritic (9,10,16-trihydroxyhexadecanoic) acid in air. J Appl Polym Sci 132: 41328
-
- Benítez JJ, Matas AJ, Heredia A (2004) Molecular characterization of the plant biopolyester cutin by AFM and spectroscopic techniques. J Struct Biol 147: 179–184 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

