Glycan:glycan interactions: High affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells
- PMID: 26676578
- PMCID: PMC4702957
- DOI: 10.1073/pnas.1421082112
Glycan:glycan interactions: High affinity biomolecular interactions that can mediate binding of pathogenic bacteria to host cells
Abstract
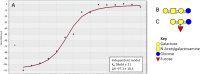
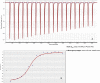
Cells from all domains of life express glycan structures attached to lipids and proteins on their surface, called glycoconjugates. Cell-to-cell contact mediated by glycan:glycan interactions have been considered to be low-affinity interactions that precede high-affinity protein-glycan or protein-protein interactions. In several pathogenic bacteria, truncation of surface glycans, lipooligosaccharide (LOS), or lipopolysaccharide (LPS) have been reported to significantly reduce bacterial adherence to host cells. Here, we show that the saccharide component of LOS/LPS have direct, high-affinity interactions with host glycans. Glycan microarrays reveal that LOS/LPS of four distinct bacterial pathogens bind to numerous host glycan structures. Surface plasmon resonance was used to determine the affinity of these interactions and revealed 66 high-affinity host-glycan:bacterial-glycan pairs with equilibrium dissociation constants (K(D)) ranging between 100 nM and 50 µM. These glycan:glycan affinity values are similar to those reported for lectins or antibodies with glycans. Cell assays demonstrated that glycan:glycan interaction-mediated bacterial adherence could be competitively inhibited by either host cell or bacterial glycans. This is the first report to our knowledge of high affinity glycan:glycan interactions between bacterial pathogens and the host. The discovery of large numbers of glycan:glycan interactions between a diverse range of structures suggests that these interactions may be important in all biological systems.
Keywords: adherence; glycoconjugates; lipooligosaccharide; lipopolysccharide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Glycoconjugates play a key role in Campylobacter jejuni Infection: interactions between host and pathogen.Front Cell Infect Microbiol. 2012 Feb 14;2:9. doi: 10.3389/fcimb.2012.00009. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919601 Free PMC article. Review.
-
Glycan-Glycan Interaction Determines Shigella Tropism toward Human T Lymphocytes.mBio. 2018 Feb 13;9(1):e02309-17. doi: 10.1128/mBio.02309-17. mBio. 2018. PMID: 29440574 Free PMC article.
-
The glycointeractome of serogroup B Neisseria meningitidis strain MC58.Sci Rep. 2017 Jul 18;7(1):5693. doi: 10.1038/s41598-017-05894-w. Sci Rep. 2017. PMID: 28720847 Free PMC article.
-
Fluorescence-based solid-phase assays to study glycan-binding protein interactions with glycoconjugates.Methods Enzymol. 2010;478:241-64. doi: 10.1016/S0076-6879(10)78012-5. Methods Enzymol. 2010. PMID: 20816484
-
Notable Aspects of Glycan-Protein Interactions.Biomolecules. 2015 Sep 1;5(3):2056-72. doi: 10.3390/biom5032056. Biomolecules. 2015. PMID: 26340640 Free PMC article. Review.
Cited by
-
The Nontypeable Haemophilus influenzae Major Adhesin Hia Is a Dual-Function Lectin That Binds to Human-Specific Respiratory Tract Sialic Acid Glycan Receptors.mBio. 2020 Nov 3;11(6):e02714-20. doi: 10.1128/mBio.02714-20. mBio. 2020. PMID: 33144377 Free PMC article.
-
Influence of Shigella flexneri 2a O Antigen Acetylation on Its Bacteriophage Sf6 Receptor Activity and Bacterial Interaction with Human Cells.J Bacteriol. 2020 Nov 19;202(24):e00363-20. doi: 10.1128/JB.00363-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 32989087 Free PMC article.
-
Interplay of Trans- and Cis-Interactions of Glycolipids in Membrane Adhesion.Front Mol Biosci. 2021 Nov 19;8:754654. doi: 10.3389/fmolb.2021.754654. eCollection 2021. Front Mol Biosci. 2021. PMID: 34869588 Free PMC article.
-
Technologies for Proteome-Wide Discovery of Extracellular Host-Pathogen Interactions.J Immunol Res. 2017;2017:2197615. doi: 10.1155/2017/2197615. Epub 2017 Feb 22. J Immunol Res. 2017. PMID: 28321417 Free PMC article. Review.
-
Cooperation of GlycoPOST and UniCarb-DR towards a comprehensive glycomics data repository workflow.Anal Bioanal Chem. 2025 Feb;417(5):1015-1023. doi: 10.1007/s00216-024-05673-3. Epub 2024 Nov 29. Anal Bioanal Chem. 2025. PMID: 39611991 Free PMC article.
References
-
- Gilboa-Garber N, Sudakevitz D. The hemagglutinating activities of Pseudomonas aeruginosa lectins PA-IL and PA-IIL exhibit opposite temperature profiles due to different receptor types. FEMS Immunol Med Microbiol. 1999;25(4):365–369. - PubMed
-
- Ilver D, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

