Tumor-suppressive microRNA-29 family inhibits cancer cell migration and invasion directly targeting LOXL2 in lung squamous cell carcinoma
- PMID: 26676674
- PMCID: PMC4725458
- DOI: 10.3892/ijo.2015.3289
Tumor-suppressive microRNA-29 family inhibits cancer cell migration and invasion directly targeting LOXL2 in lung squamous cell carcinoma
Abstract
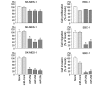
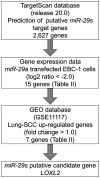
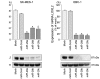
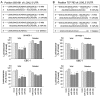
Lung cancer remains the most frequent cause of cancer-related death in developed countries. A recent molecular-targeted strategy has contributed to improvement of the remarkable effect of adenocarcinoma of the lung. However, such treatment has not been developed for squamous cell carcinoma (SCC) of the disease. Our recent studies of microRNA (miRNA) expression signatures of human cancers showed that the microRNA-29 family (miR‑29a, miR‑29b and miR‑29c) significantly reduced cancer tissues compared to normal tissues. These findings suggest that miR‑29s act as tumor-suppressors by targeting several oncogenic genes. The aim of the study was to investigate the functional significance of miR‑29s in lung SCC and to identify miR‑29s modulating molecular targets in lung SCC cells. Restoration of all mature members of the miR‑29s inhibited cancer cell migration and invasion. Gene expression data combined in silico analysis and luciferase reporter assays demonstrated that the lysyl oxidase-like 2 (LOXL2) gene was a direct regulator of tumor‑suppressive miR‑29s. Moreover, overexpressed LOXL2 was confirmed in lung SCC clinical specimens, and silencing of LOXL2 inhibited cancer cell migration and invasion in lung SCC cell lines. Our present data suggested that loss of tumor-suppressive miR‑29s enhanced cancer cell invasion in lung SCC through direct regulation of oncogenic LOXL2. Elucidation of the novel lung SCC molecular pathways and targets regulated by tumor-suppressive miR‑29s will provide new insights into the potential mechanisms of oncogenesis and metastasis of the disease.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

