Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds
- PMID: 26676860
- PMCID: PMC4681752
- DOI: 10.1007/s10856-015-5627-8
Optimisation of UV irradiation as a binding site conserving method for crosslinking collagen-based scaffolds
Abstract
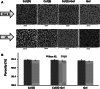
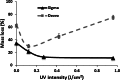
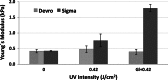
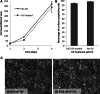
Short wavelength (λ = 254 nm) UV irradiation was evaluated over a range of intensities (0.06 to 0.96 J/cm(2)) as a means of cross-linking collagen- and gelatin-based scaffolds, to tailor their material characteristics whilst retaining biological functionality. Zero-link carbodiimide treatments are commonly applied to collagen-based materials, forming cross-links from carboxylate anions (for example the acidic E of GFOGER) that are an essential part of integrin binding sites on collagen. Cross-linking these amino acids therefore disrupts the bioactivity of collagen. In contrast, UV irradiation forms bonds from less important aromatic tyrosine and phenylalanine residues. We therefore hypothesised that UV cross-linking would not compromise collagen cell reactivity. Here, highly porous (~99 %) isotropic, collagen-based scaffolds were produced via ice-templating. A series of scaffolds (pore diameters ranging from 130-260 μm) with ascending stability in water was made from gelatin, two different sources of collagen I, or blends of these materials. Glucose, known to aid UV crosslinking of collagen, was added to some lower-stability formulations. These scaffolds were exposed to different doses of UV irradiation, and the scaffold morphology, dissolution stability in water, resistance to compression and cell reactivity was assessed. Stabilisation in aqueous media varied with both the nature of the collagen-based material employed and the UV intensity. Scaffolds made from the most stable materials showed the greatest stability after irradiation, although the levels of cross-linking in all cases were relatively low. Scaffolds made from pure collagen from the two different sources showed different optimum levels of irradiation, suggesting altered balance between stabilisation from cross-linking and destabilisation from denaturation. The introduction of glucose into the scaffold enhanced the efficacy of UV cross-linking. Finally, as hypothesized, cell attachment, spreading and proliferation on collagen materials were unaffected by UV cross-linking. UV irradiation may therefore be used to provide relatively low level cross-linking of collagen without loss of biological functionality.
Figures







Similar articles
-
Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics.Acta Biomater. 2015 Oct;25:131-142. doi: 10.1016/j.actbio.2015.07.034. Epub 2015 Jul 26. Acta Biomater. 2015. PMID: 26213371 Free PMC article.
-
Comparison of the properties of collagen-chitosan scaffolds after γ-ray irradiation and carbodiimide cross-linking.J Biomater Sci Polym Ed. 2016 Jul;27(10):937-53. doi: 10.1080/09205063.2016.1169478. Epub 2016 Apr 27. J Biomater Sci Polym Ed. 2016. PMID: 27122297
-
Impact of UV- and carbodiimide-based crosslinking on the integrin-binding properties of collagen-based materials.Acta Biomater. 2019 Dec;100:280-291. doi: 10.1016/j.actbio.2019.09.046. Epub 2019 Oct 2. Acta Biomater. 2019. PMID: 31586463
-
Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering.J Mech Behav Biomed Mater. 2012 Jun;10:62-74. doi: 10.1016/j.jmbbm.2012.02.028. Epub 2012 Mar 16. J Mech Behav Biomed Mater. 2012. PMID: 22520419
-
Chemical stabilisation of collagen as a biomimetic.ScientificWorldJournal. 2003 Mar 24;3:138-55. doi: 10.1100/tsw.2003.13. ScientificWorldJournal. 2003. PMID: 12806126 Free PMC article. Review.
Cited by
-
Expanding limits of artificial enzymes: unprecedented catalysis by an oxidase nanozyme in activating a structural protein for covalent crosslinking and conferring remarkable proteolytic resistance.Chem Sci. 2024 Aug 20;15(36):14726-38. doi: 10.1039/d4sc03767g. Online ahead of print. Chem Sci. 2024. PMID: 39176248 Free PMC article.
-
Ultra-Rapid and Specific Gelation of Collagen Molecules for Transparent and Tough Gels by Transition Metal Complexation.Adv Sci (Weinh). 2023 Oct;10(30):e2302637. doi: 10.1002/advs.202302637. Epub 2023 Sep 11. Adv Sci (Weinh). 2023. PMID: 37697642 Free PMC article.
-
Recent Advancements in Marine Collagen: Exploring New Sources, Processing Approaches, and Nutritional Applications.Mar Drugs. 2025 Apr 28;23(5):190. doi: 10.3390/md23050190. Mar Drugs. 2025. PMID: 40422780 Free PMC article. Review.
-
Surface Characterization of Skin Substitute Materials.Skin Res Technol. 2025 Jul;31(7):e70187. doi: 10.1111/srt.70187. Skin Res Technol. 2025. PMID: 40583047 Free PMC article.
-
Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration.Front Bioeng Biotechnol. 2021 Apr 27;9:644595. doi: 10.3389/fbioe.2021.644595. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33987173 Free PMC article. Review.
References
-
- Harley BAC, Gibson LJ. In vivo and in vitro applications of collagen-GAG scaffolds. Chem Eng J. 2008;137:102–121. doi: 10.1016/j.cej.2007.09.009. - DOI
-
- Chen Q-Z, Harding SE, Ali NN, Lyon AR, Boccaccini AR. Biomaterials in cardiac tissue engineering: ten years of research survey. Mater Sci Eng R Rep. 2008;59:1–37. doi: 10.1016/j.mser.2007.08.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

