Interleukin-17A as a Biomarker for Bovine Tuberculosis
- PMID: 26677202
- PMCID: PMC4744917
- DOI: 10.1128/CVI.00637-15
Interleukin-17A as a Biomarker for Bovine Tuberculosis
Abstract
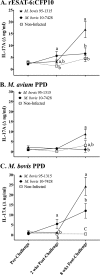
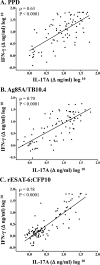
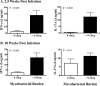
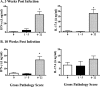
T helper 17 (Th17)-associated cytokines are integral to the immune responses to tuberculosis, initiating both protective and harmful inflammatory responses. The aim of the present study was to evaluate applied aspects of interleukin-17 (IL-17) biology in the context of Mycobacterium bovis infection of cattle. Using transcriptome sequencing (RNA-Seq), numerous Th17-associated cytokine genes (including IL-17A, IL-17F, IL-22, IL-19, and IL-27) were upregulated >9-fold in response to purified protein derivative stimulation of peripheral blood mononuclear cells from experimentally M. bovis-infected cattle. Protective vaccines elicited IL-17A, IL-17F, IL-22, and IL-27 responses. Reduced IL-17A responses by vaccine recipients, compared to nonvaccinated animals, at 2.5 weeks after M. bovis challenge correlated with reduced disease burdens. Additionally, IL-17A and interferon gamma (IFN-γ) responses were highly correlated and exhibited similar diagnostic capacities. The present findings support the use of Th17-associated cytokines as biomarkers of infection and protection in the immune responses to bovine tuberculosis.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Use of antigen-specific interleukin-2 to differentiate between cattle vaccinated with Mycobacterium bovis BCG and cattle infected with M. bovis.Clin Vaccine Immunol. 2014 Jan;21(1):39-45. doi: 10.1128/CVI.00522-13. Epub 2013 Oct 30. Clin Vaccine Immunol. 2014. PMID: 24173026 Free PMC article.
-
Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens.Clin Diagn Lab Immunol. 1999 Jan;6(1):1-5. doi: 10.1128/CDLI.6.1.1-5.1999. Clin Diagn Lab Immunol. 1999. PMID: 9874655 Free PMC article.
-
Increased pulmonary tumor necrosis factor alpha, interleukin-6 (IL-6), and IL-17A responses compensate for decreased gamma interferon production in anti-IL-12 autovaccine-treated, Mycobacterium bovis BCG-vaccinated mice.Clin Vaccine Immunol. 2011 Jan;18(1):95-104. doi: 10.1128/CVI.00352-10. Epub 2010 Nov 17. Clin Vaccine Immunol. 2011. PMID: 21084465 Free PMC article.
-
Identification of immune response correlates for protection against bovine tuberculosis.Vet Immunol Immunopathol. 2005 Oct 18;108(1-2):45-51. doi: 10.1016/j.vetimm.2005.08.002. Vet Immunol Immunopathol. 2005. PMID: 16140388 Review.
-
Development of vaccines to control bovine tuberculosis in cattle and relationship to vaccine development for other intracellular pathogens.Int J Parasitol. 2003 May;33(5-6):555-66. doi: 10.1016/s0020-7519(03)00060-2. Int J Parasitol. 2003. PMID: 12782055 Review.
Cited by
-
Biomarkers for Detecting Resilience against Mycobacterial Disease in Animals.Infect Immun. 2019 Dec 17;88(1):e00401-19. doi: 10.1128/IAI.00401-19. Print 2019 Dec 17. Infect Immun. 2019. PMID: 31527123 Free PMC article. Review.
-
Mycobacterium avium Subspecies paratuberculosis Drives an Innate Th17-Like T Cell Response Regardless of the Presence of Antigen-Presenting Cells.Front Vet Sci. 2020 Mar 17;7:108. doi: 10.3389/fvets.2020.00108. eCollection 2020. Front Vet Sci. 2020. PMID: 32258066 Free PMC article.
-
Type 3 immunity: a perspective for the defense of the mammary gland against infections.Vet Res. 2020 Oct 15;51(1):129. doi: 10.1186/s13567-020-00852-3. Vet Res. 2020. PMID: 33059767 Free PMC article.
-
Use of fecal volatile organic compound analysis to discriminate between non-vaccinated and BCG-Vaccinated cattle prior to and after Mycobacterium bovis challenge.PLoS One. 2017 Jul 7;12(7):e0179914. doi: 10.1371/journal.pone.0179914. eCollection 2017. PLoS One. 2017. PMID: 28686691 Free PMC article.
-
Establishment a real-time reverse transcription PCR based on host biomarkers for the detection of the subclinical cases of Mycobacterium avium subsp. paratuberculosis.PLoS One. 2017 May 25;12(5):e0178336. doi: 10.1371/journal.pone.0178336. eCollection 2017. PLoS One. 2017. PMID: 28542507 Free PMC article.
References
-
- Palmer MV. 2013. Mycobacterium bovis: characteristics of wildlife reservoir hosts. Transbound Emerg Dis 60(Suppl 1):S1–S13. - PubMed
-
- Schiller I, Oesch B, Vordermeier HM, Palmer MV, Harris BN, Orloski KA, Buddle BM, Thacker TC, Lyashchenko KP, Waters WR. 2010. Bovine tuberculosis: a review of current and emerging diagnostic techniques in view of their relevance for disease control and eradication. Transbound Emerg Dis 57:205–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

