Phototransduction Influences Metabolic Flux and Nucleotide Metabolism in Mouse Retina
- PMID: 26677218
- PMCID: PMC4813492
- DOI: 10.1074/jbc.M115.698985
Phototransduction Influences Metabolic Flux and Nucleotide Metabolism in Mouse Retina
Abstract
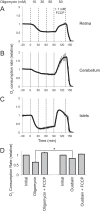
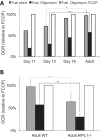
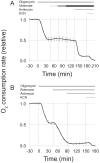
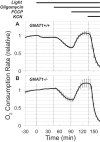
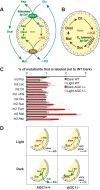
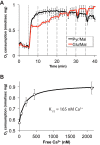
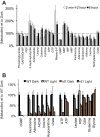
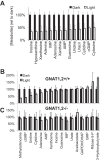
Production of energy in a cell must keep pace with demand. Photoreceptors use ATP to maintain ion gradients in darkness, whereas in light they use it to support phototransduction. Matching production with consumption can be accomplished by coupling production directly to consumption. Alternatively, production can be set by a signal that anticipates demand. In this report we investigate the hypothesis that signaling through phototransduction controls production of energy in mouse retinas. We found that respiration in mouse retinas is not coupled tightly to ATP consumption. By analyzing metabolic flux in mouse retinas, we also found that phototransduction slows metabolic flux through glycolysis and through intermediates of the citric acid cycle. We also evaluated the relative contributions of regulation of the activities of α-ketoglutarate dehydrogenase and the aspartate-glutamate carrier 1. In addition, a comprehensive analysis of the retinal metabolome showed that phototransduction also influences steady-state concentrations of 5'-GMP, ribose-5-phosphate, ketone bodies, and purines.
Keywords: anaerobic metabolism; calcium; mitochondria; photoreceptor; phototransduction; retina.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Autophagy-mediated catabolism of visual transduction proteins prevents retinal degeneration.Autophagy. 2016 Dec;12(12):2439-2450. doi: 10.1080/15548627.2016.1238553. Epub 2016 Oct 18. Autophagy. 2016. PMID: 27753525 Free PMC article.
-
Opsin, G-protein and 48-kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light/dark cycling of mRNAs.Exp Eye Res. 1988 Sep;47(3):369-90. doi: 10.1016/0014-4835(88)90049-8. Exp Eye Res. 1988. PMID: 2846333
-
Separation of photoreceptor cell compartments in mouse retina for protein analysis.Mol Neurodegener. 2017 Apr 11;12(1):28. doi: 10.1186/s13024-017-0171-2. Mol Neurodegener. 2017. PMID: 28399904 Free PMC article.
-
Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones.Adv Exp Med Biol. 2002;514:179-203. doi: 10.1007/978-1-4615-0121-3_11. Adv Exp Med Biol. 2002. PMID: 12596922 Review.
-
Multiple Roles of cAMP in Vertebrate Retina.Cells. 2023 Apr 14;12(8):1157. doi: 10.3390/cells12081157. Cells. 2023. PMID: 37190066 Free PMC article. Review.
Cited by
-
Inhibition of Mitochondrial Respiration Impairs Nutrient Consumption and Metabolite Transport in Human Retinal Pigment Epithelium.J Proteome Res. 2021 Jan 1;20(1):909-922. doi: 10.1021/acs.jproteome.0c00690. Epub 2020 Sep 25. J Proteome Res. 2021. PMID: 32975122 Free PMC article.
-
Pyruvate kinase M2 regulates photoreceptor structure, function, and viability.Cell Death Dis. 2018 Feb 14;9(2):240. doi: 10.1038/s41419-018-0296-4. Cell Death Dis. 2018. PMID: 29445082 Free PMC article.
-
Mouse Models of Inherited Retinal Degeneration with Photoreceptor Cell Loss.Cells. 2020 Apr 10;9(4):931. doi: 10.3390/cells9040931. Cells. 2020. PMID: 32290105 Free PMC article. Review.
-
Metabolism in the Zebrafish Retina.J Dev Biol. 2021 Mar 15;9(1):10. doi: 10.3390/jdb9010010. J Dev Biol. 2021. PMID: 33804189 Free PMC article. Review.
-
Diabetic Retinopathy: The Role of Mitochondria in the Neural Retina and Microvascular Disease.Antioxidants (Basel). 2020 Sep 23;9(10):905. doi: 10.3390/antiox9100905. Antioxidants (Basel). 2020. PMID: 32977483 Free PMC article. Review.
References
-
- Warburg O., Posener K., and Negelein E. (1924) On the metabolism of carcinoma cells. Biochemische Zeitschrift 152, 309–344
-
- Krebs H. A. (1927) On the metabolism of the retina. Biochemische Zeitschrift 189, 57–59
-
- Lunt S. Y., and Vander Heiden M. G. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

