Mitochondrial Sulfide Quinone Oxidoreductase Prevents Activation of the Unfolded Protein Response in Hydrogen Sulfide
- PMID: 26677221
- PMCID: PMC4777863
- DOI: 10.1074/jbc.M115.697102
Mitochondrial Sulfide Quinone Oxidoreductase Prevents Activation of the Unfolded Protein Response in Hydrogen Sulfide
Abstract
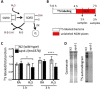
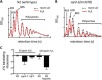
Hydrogen sulfide (H2S) is an endogenously produced gaseous molecule with important roles in cellular signaling. In mammals, exogenous H2S improves survival of ischemia/reperfusion. We have previously shown that exposure to H2S increases the lifespan and thermotolerance in Caenorhabditis elegans, and improves protein homeostasis in low oxygen. The mitochondrial SQRD-1 (sulfide quinone oxidoreductase) protein is a highly conserved enzyme involved in H2S metabolism. SQRD-1 is generally considered important to detoxify H2S. Here, we show that SQRD-1 is also required to maintain protein translation in H2S. In sqrd-1 mutant animals, exposure to H2S leads to phosphorylation of eIF2α and inhibition of protein synthesis. In contrast, global protein translation is not altered in wild-type animals exposed to lethally high H2S or in hif-1(ia04) mutants that die when exposed to low H2S. We demonstrate that both gcn-2 and pek-1 kinases are involved in the H2S-induced phosphorylation of eIF2α. Both ER and mitochondrial stress responses are activated in sqrd-1 mutant animals exposed to H2S, but not in wild-type animals. We speculate that SQRD-1 activity in H2S may coordinate proteostasis responses in multiple cellular compartments.
Keywords: Caenorhabditis elegans (C. elegans); eukaryotic initiation factor 2 (eIF2); hydrogen sulfide; proteostasis; translation initiation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
The response of Caenorhabditis elegans to hydrogen sulfide and hydrogen cyanide.Genetics. 2011 Oct;189(2):521-32. doi: 10.1534/genetics.111.129841. Epub 2011 Aug 11. Genetics. 2011. PMID: 21840852 Free PMC article.
-
Rhodoquinone-dependent electron transport chain is essential for Caenorhabditis elegans survival in hydrogen sulfide environments.J Biol Chem. 2024 Sep;300(9):107708. doi: 10.1016/j.jbc.2024.107708. Epub 2024 Aug 22. J Biol Chem. 2024. PMID: 39178951 Free PMC article.
-
Caenorhabditis elegans HIF-1 Is Broadly Required for Survival in Hydrogen Sulfide.G3 (Bethesda). 2017 Nov 6;7(11):3699-3704. doi: 10.1534/g3.117.300146. G3 (Bethesda). 2017. PMID: 28889102 Free PMC article.
-
Cellular Homeostasis and Aging.Annu Rev Biochem. 2016 Jun 2;85:1-4. doi: 10.1146/annurev-biochem-011116-110806. Epub 2016 Apr 6. Annu Rev Biochem. 2016. PMID: 27050288 Review.
-
Hydrogen sulfide in pharmacology and medicine--An update.Pharmacol Rep. 2015 Jun;67(3):647-58. doi: 10.1016/j.pharep.2015.01.005. Epub 2015 Jan 22. Pharmacol Rep. 2015. PMID: 25933982 Review.
Cited by
-
Xenobiotic metabolism and transport in Caenorhabditis elegans.J Toxicol Environ Health B Crit Rev. 2021 Feb 17;24(2):51-94. doi: 10.1080/10937404.2021.1884921. Epub 2021 Feb 22. J Toxicol Environ Health B Crit Rev. 2021. PMID: 33616007 Free PMC article. Review.
-
Hydrogen Sulfide Oxidation by Sulfide Quinone Oxidoreductase.Chembiochem. 2021 Mar 16;22(6):949-960. doi: 10.1002/cbic.202000661. Epub 2020 Nov 17. Chembiochem. 2021. PMID: 33080111 Free PMC article. Review.
-
Whole genome profiling of short-term hypoxia induced genes and identification of HIF-1 binding sites provide insights into HIF-1 function in Caenorhabditis elegans.bioRxiv [Preprint]. 2023 Nov 17:2023.11.15.567310. doi: 10.1101/2023.11.15.567310. bioRxiv. 2023. Update in: PLoS One. 2024 May 14;19(5):e0295094. doi: 10.1371/journal.pone.0295094. PMID: 38014054 Free PMC article. Updated. Preprint.
-
Perspective: Modulating the integrated stress response to slow aging and ameliorate age-related pathology.Nat Aging. 2021 Sep;1(9):760-768. doi: 10.1038/s43587-021-00112-9. Epub 2021 Sep 13. Nat Aging. 2021. PMID: 35146440 Free PMC article.
-
Dietary and Endocrine Regulation of Endogenous Hydrogen Sulfide Production: Implications for Longevity.Antioxid Redox Signal. 2018 Jun 1;28(16):1483-1502. doi: 10.1089/ars.2017.7434. Antioxid Redox Signal. 2018. PMID: 29634343 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

