Identification of the Glycosaminoglycan Binding Site of Interleukin-10 by NMR Spectroscopy
- PMID: 26677224
- PMCID: PMC4742770
- DOI: 10.1074/jbc.M115.681759
Identification of the Glycosaminoglycan Binding Site of Interleukin-10 by NMR Spectroscopy
Abstract
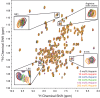
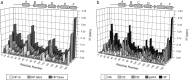
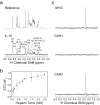
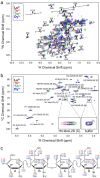
The biological function of interleukin-10 (IL-10), a pleiotropic cytokine with an essential role in inflammatory processes, is known to be affected by glycosaminoglycans (GAGs). GAGs are highly negatively charged polysaccharides and integral components of the extracellular matrix with important functions in the biology of many growth factors and cytokines. The molecular mechanism of the IL-10/GAG interaction is unclear. In particular, experimental evidence about IL-10/GAG binding sites is lacking, despite its importance for understanding the biological role of the interaction. Here, we report the experimental determination of a GAG binding site of IL-10. Although no co-crystal structure of the IL-10·GAG complex could be obtained, its structural characterization was possible by NMR spectroscopy. Chemical shift perturbations of IL-10 induced by GAG binding were used to narrow down the location of the binding site and to assess the affinity for different GAG molecules. Subsequent observation of NMR pseudocontact shifts of IL-10 and its heparin ligand, as induced by a protein-attached lanthanide spin label, provided structural restraints for the protein·ligand complex. Using these restraints, pseudocontact shift-based rigid body docking together with molecular dynamics simulations yielded a GAG binding model. The heparin binding site is located at the C-terminal end of helix D and the adjacent DE loop and coincides with a patch of positively charged residues involving arginines 102, 104, 106, and 107 and lysines 117 and 119. This study represents the first experimental characterization of the IL-10·GAG complex structure and provides the starting point for revealing the biological significance of the interaction of IL-10 with GAGs.
Keywords: carbohydrate function; extracellular matrix; glycosaminoglycan; heparin; interleukin-10; nuclear magnetic resonance (NMR); paramagnetic NMR; pseudocontact shifts.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., and O'Garra A. (1991) IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146, 3444–3451 - PubMed
-
- Ding L., and Shevach E. M. (1992) IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J. Immunol. 148, 3133–3139 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

