Ras Regulates Rb via NORE1A
- PMID: 26677227
- PMCID: PMC4742771
- DOI: 10.1074/jbc.M115.697557
Ras Regulates Rb via NORE1A
Abstract
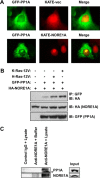
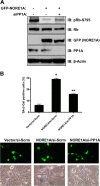
Mutations in the Ras oncogene are one of the most frequent events in human cancer. Although Ras regulates numerous growth-promoting pathways to drive transformation, it can paradoxically promote an irreversible cell cycle arrest known as oncogene-induced senescence. Although senescence has clearly been implicated as a major defense mechanism against tumorigenesis, the mechanisms by which Ras can promote such a senescent phenotype remain poorly defined. We have shown recently that the Ras death effector NORE1A plays a critical role in promoting Ras-induced senescence and connects Ras to the regulation of the p53 tumor suppressor. We now show that NORE1A also connects Ras to the regulation of a second major prosenescent tumor suppressor, the retinoblastoma (Rb) protein. We show that Ras induces the formation of a complex between NORE1A and the phosphatase PP1A, promoting the activation of the Rb tumor suppressor by dephosphorylation. Furthermore, suppression of Rb reduces NORE1A senescence activity. These results, together with our previous findings, suggest that NORE1A acts as a critical tumor suppressor node, linking Ras to both the p53 and the Rb pathways to drive senescence.
Keywords: NORE1A; RASSF; Ras protein; cancer; cellular senescence; protein phosphorylation; retinoblastoma protein (pRb, RB).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
The role of the NORE1A tumor suppressor in Oncogene-Induced Senescence.Cancer Lett. 2017 Aug 1;400:30-36. doi: 10.1016/j.canlet.2017.04.030. Epub 2017 Apr 26. Cancer Lett. 2017. PMID: 28455242 Free PMC article. Review.
-
NORE1A is a Ras senescence effector that controls the apoptotic/senescent balance of p53 via HIPK2.J Cell Biol. 2015 Mar 16;208(6):777-89. doi: 10.1083/jcb.201408087. J Cell Biol. 2015. PMID: 25778922 Free PMC article.
-
NORE1A is a double barreled Ras senescence effector that activates p53 and Rb.Cell Cycle. 2016 Sep;15(17):2263-4. doi: 10.1080/15384101.2016.1152431. Epub 2016 Feb 26. Cell Cycle. 2016. PMID: 26919075 Free PMC article.
-
NORE1A tumor suppressor candidate modulates p21CIP1 via p53.Cancer Res. 2009 Jun 1;69(11):4629-37. doi: 10.1158/0008-5472.CAN-08-3672. Epub 2009 May 12. Cancer Res. 2009. PMID: 19435914 Free PMC article.
-
RB functions as a key regulator of senescence and tumor suppression.Semin Cancer Biol. 2025 Feb;109:1-7. doi: 10.1016/j.semcancer.2024.11.004. Epub 2024 Dec 13. Semin Cancer Biol. 2025. PMID: 39675647 Review.
Cited by
-
The Influence of Oncogenic RAS on Chemotherapy and Radiotherapy Resistance Through DNA Repair Pathways.Front Cell Dev Biol. 2022 Mar 11;10:751367. doi: 10.3389/fcell.2022.751367. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35359456 Free PMC article. Review.
-
BRCA1 and NORE1A Form a Her2/Ras Regulated Tumor Suppressor Complex Modulating Senescence.Cancers (Basel). 2023 Aug 16;15(16):4133. doi: 10.3390/cancers15164133. Cancers (Basel). 2023. PMID: 37627161 Free PMC article.
-
Ras signaling through RASSF proteins.Semin Cell Dev Biol. 2016 Oct;58:86-95. doi: 10.1016/j.semcdb.2016.06.007. Epub 2016 Jun 8. Semin Cell Dev Biol. 2016. PMID: 27288568 Free PMC article. Review.
-
Engineered variants of the Ras effector protein RASSF5 (NORE1A) promote anticancer activities in lung adenocarcinoma.J Biol Chem. 2021 Dec;297(6):101353. doi: 10.1016/j.jbc.2021.101353. Epub 2021 Oct 27. J Biol Chem. 2021. PMID: 34717958 Free PMC article.
-
Proteomics Analysis Reveals Novel RASSF2 Interaction Partners.Cancers (Basel). 2016 Mar 16;8(3):37. doi: 10.3390/cancers8030037. Cancers (Basel). 2016. PMID: 26999212 Free PMC article.
References
-
- DeNicola G. M., and Tuveson D. A. (2009) RAS in cellular transformation and senescence. Eur. J. Cancer 45, 211–216 - PubMed
-
- Campbell P. M., and Der C. J. (2004) Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin. Cancer Biol. 14, 105–114 - PubMed
-
- Malumbres M., and Barbacid M. (2003) RAS oncogenes: the first 30 years. Nat. Rev. Cancer 3, 459–465 - PubMed
-
- Serrano M., Lin A. W., McCurrach M. E., Beach D., and Lowe S. W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

