A Unique Tool for Cellular Structural Biology: In-cell NMR
- PMID: 26677229
- PMCID: PMC4759160
- DOI: 10.1074/jbc.R115.643247
A Unique Tool for Cellular Structural Biology: In-cell NMR
Abstract
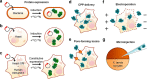
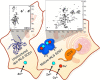
Conventional structural and chemical biology approaches are applied to macromolecules extrapolated from their native context. When this is done, important structural and functional features of macromolecules, which depend on their native network of interactions within the cell, may be lost. In-cell nuclear magnetic resonance is a branch of biomolecular NMR spectroscopy that allows macromolecules to be analyzed in living cells, at the atomic level. In-cell NMR can be applied to several cellular systems to obtain biologically relevant structural and functional information. Here we summarize the existing approaches and focus on the applications to protein folding, interactions, and post-translational modifications.
Keywords: cell compartmentalization; intracellular processing; molecular cell biology; nuclear magnetic resonance (NMR); post-translational modification (PTM); protein folding; structural biology.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Towards understanding cellular structure biology: In-cell NMR.Biochim Biophys Acta Proteins Proteom. 2017 May;1865(5):547-557. doi: 10.1016/j.bbapap.2017.02.018. Epub 2017 Mar 1. Biochim Biophys Acta Proteins Proteom. 2017. PMID: 28257994 Review.
-
Real-time NMR monitoring of biological activities in complex physiological environments.Curr Opin Struct Biol. 2015 Jun;32:39-47. doi: 10.1016/j.sbi.2015.02.003. Epub 2015 Feb 27. Curr Opin Struct Biol. 2015. PMID: 25727665 Review.
-
NMR and semi-synthesis in synergy to study protein regulation.J Struct Biol. 2025 Jun;217(2):108192. doi: 10.1016/j.jsb.2025.108192. Epub 2025 Mar 13. J Struct Biol. 2025. PMID: 40089044 Review.
-
In-Cell NMR in Human Cells: Direct Protein Expression Allows Structural Studies of Protein Folding and Maturation.Acc Chem Res. 2018 Jun 19;51(6):1550-1557. doi: 10.1021/acs.accounts.8b00147. Epub 2018 Jun 5. Acc Chem Res. 2018. PMID: 29869502
-
Quo Vadis Biomolecular NMR Spectroscopy?Int J Mol Sci. 2019 Mar 14;20(6):1278. doi: 10.3390/ijms20061278. Int J Mol Sci. 2019. PMID: 30875725 Free PMC article. Review.
Cited by
-
On the necessity of an integrative approach to understand protein structural dynamics.J Zhejiang Univ Sci B. 2019 Jun;20(6):496-502. doi: 10.1631/jzus.B1900135. J Zhejiang Univ Sci B. 2019. PMID: 31090275 Free PMC article. Review.
-
Introduction to the Minireview Series on Modern Technologies for In-cell Biochemistry.J Biol Chem. 2016 Feb 19;291(8):3757-8. doi: 10.1074/jbc.R115.709444. Epub 2015 Dec 16. J Biol Chem. 2016. PMID: 26677225 Free PMC article. Review.
-
In-Cell NMR: Analysis of Protein-Small Molecule Interactions, Metabolic Processes, and Protein Phosphorylation.Int J Mol Sci. 2019 Jan 17;20(2):378. doi: 10.3390/ijms20020378. Int J Mol Sci. 2019. PMID: 30658393 Free PMC article. Review.
-
NMR as a Tool to Investigate the Processes of Mitochondrial and Cytosolic Iron-Sulfur Cluster Biosynthesis.Molecules. 2018 Aug 31;23(9):2213. doi: 10.3390/molecules23092213. Molecules. 2018. PMID: 30200358 Free PMC article. Review.
-
The role of native cysteine residues in the oligomerization of KCNQ1 channels.Biochem Biophys Res Commun. 2023 Jun 4;659:34-39. doi: 10.1016/j.bbrc.2023.03.082. Epub 2023 Mar 31. Biochem Biophys Res Commun. 2023. PMID: 37031592 Free PMC article.
References
-
- Serber Z., Keatinge-Clay A. T., Ledwidge R., Kelly A. E., Miller S. M., and Dötsch V. (2001) High-resolution macromolecular NMR spectroscopy inside living cells. J. Am. Chem. Soc. 123, 2446–2447 - PubMed
-
- Serber Z., Ledwidge R., Miller S. M., and Dötsch V. (2001) Evaluation of parameters critical to observing proteins inside living Escherichia coli by in-cell NMR spectroscopy. J. Am. Chem. Soc. 123, 8895–8901 - PubMed
-
- Serber Z., Straub W., Corsini L., Nomura A. M., Shimba N., Craik C. S., Ortiz de Montellano P., and Dötsch V. (2004) Methyl groups as probes for proteins and complexes in in-cell NMR experiments. J. Am. Chem. Soc. 126, 7119–7125 - PubMed
-
- Ye Y., Liu X., Zhang Z., Wu Q., Jiang B., Jiang L., Zhang X., Liu M., Pielak G. J., and Li C. (2013) 19F NMR spectroscopy as a probe of cytoplasmic viscosity and weak protein interactions in living cells. Chemistry 19, 12705–12710 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

