Reduced Adult Hippocampal Neurogenesis and Cognitive Impairments following Prenatal Treatment of the Antiepileptic Drug Valproic Acid
- PMID: 26677766
- PMCID: PMC4682151
- DOI: 10.1016/j.stemcr.2015.10.012
Reduced Adult Hippocampal Neurogenesis and Cognitive Impairments following Prenatal Treatment of the Antiepileptic Drug Valproic Acid
Abstract
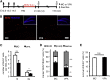
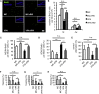
Prenatal exposure to valproic acid (VPA), an established antiepileptic drug, has been reported to impair postnatal cognitive function in children born to VPA-treated epileptic mothers. However, how these defects arise and how they can be overcome remain unknown. Using mice, we found that comparable postnatal cognitive functional impairment is very likely correlated to the untimely enhancement of embryonic neurogenesis, which led to depletion of the neural precursor cell pool and consequently a decreased level of adult neurogenesis in the hippocampus. Moreover, hippocampal neurons in the offspring of VPA-treated mice showed abnormal morphology and activity. Surprisingly, these impairments could be ameliorated by voluntary running. Our study suggests that although prenatal exposure to antiepileptic drugs such as VPA may have detrimental effects that persist until adulthood, these effects may be offset by a simple physical activity such as running.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Alvarez-Buylla A., Lim D.A. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
-
- Balasubramaniyan V., Boddeke E., Bakels R., Küst B., Kooistra S., Veneman A., Copray S. Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cells. Neuroscience. 2006;143:939–951. - PubMed
-
- Battino D., Tomson T. Management of epilepsy during pregnancy. Drugs. 2007;67:2727–2746. - PubMed
-
- Bekinschtein P., Oomen C.A., Saksida L.M., Bussey T.J. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin. Cell Dev. Biol. 2011;22:536–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

