TRPV4 associates environmental temperature and sex determination in the American alligator
- PMID: 26677944
- PMCID: PMC4683465
- DOI: 10.1038/srep18581
TRPV4 associates environmental temperature and sex determination in the American alligator
Abstract
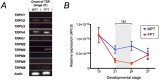
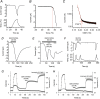
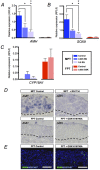
Temperature-dependent sex determination (TSD), commonly found among reptiles, is a sex determination mode in which the incubation temperature during a critical temperature sensitive period (TSP) determines sexual fate of the individual rather than the individual's genotypic background. In the American alligator (Alligator mississippiensis), eggs incubated during the TSP at 33 °C (male producing temperature: MPT) yields male offspring, whereas incubation temperatures below 30 °C (female producing temperature: FPT) lead to female offspring. However, many of the details of the underlying molecular mechanism remains elusive, and the molecular link between environmental temperature and sex determination pathway is yet to be elucidated. Here we show the alligator TRPV4 ortholog (AmTRPV4) to be activated at temperatures proximate to the TSD-related temperature in alligators, and using pharmacological exposure, we show that AmTRPV4 channel activity affects gene expression patterns associated with male differentiation. This is the first experimental demonstration of a link between a well-described thermo-sensory mechanism, TRPV4 channel, and its potential role in regulation of TSD in vertebrates, shedding unique new light on the elusive TSD molecular mechanism.
Figures

References
-
- Bull J. J. Sex determination in reptiles. Q. Rev. Biol. 55, 3–21 (1980).
-
- Charnier M. [Action of temperature on the sex ratio in the Agama agama (Agamidae, Lacertilia) embryo]. C. R. Seances Soc. Biol. Fil. 160, 620–622 (1966). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

