Human Enteroids as a Model of Upper Small Intestinal Ion Transport Physiology and Pathophysiology
- PMID: 26677983
- PMCID: PMC4766025
- DOI: 10.1053/j.gastro.2015.11.047
Human Enteroids as a Model of Upper Small Intestinal Ion Transport Physiology and Pathophysiology
Abstract
Background & aims: Human intestinal crypt-derived enteroids are a model of intestinal ion transport that require validation by comparison with cell culture and animal models. We used human small intestinal enteroids to study neutral Na(+) absorption and stimulated fluid and anion secretion under basal and regulated conditions in undifferentiated and differentiated cultures to show their functional relevance to ion transport physiology and pathophysiology.
Methods: Human intestinal tissue specimens were obtained from an endoscopic biopsy or surgical resections performed at Johns Hopkins Hospital. Crypts were isolated, enteroids were propagated in culture, induced to undergo differentiation, and transduced with lentiviral vectors. Crypt markers, surface cell enzymes, and membrane ion transporters were characterized using quantitative reverse-transcription polymerase chain reaction, immunoblot, or immunofluorescence analyses. We used multiphoton and time-lapse confocal microscopy to monitor intracellular pH and luminal dilatation in enteroids under basal and regulated conditions.
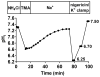
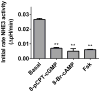
Results: Enteroids differentiated upon withdrawal of WNT3A, yielding decreased crypt markers and increased villus-like characteristics. Na(+)/H(+) exchanger 3 activity was similar in undifferentiated and differentiated enteroids, and was affected by known inhibitors, second messengers, and bacterial enterotoxins. Forskolin-induced swelling was completely dependent on cystic fibrosis transmembrane conductance regulator and partially dependent on Na(+)/H(+) exchanger 3 and Na(+)/K(+)/2Cl(-) cotransporter 1 inhibition in undifferentiated and differentiated enteroids. Increases in cyclic adenosine monophosphate with forskolin caused enteroid intracellular acidification in HCO3(-)-free buffer. Cyclic adenosine monophosphate-induced enteroid intracellular pH acidification as part of duodenal HCO3(-) secretion appears to require cystic fibrosis transmembrane conductance regulator and electrogenic Na(+)/HCO3(-) cotransporter 1.
Conclusions: Undifferentiated or crypt-like, and differentiated or villus-like, human enteroids represent distinct points along the crypt-villus axis; they can be used to characterize electrolyte transport processes along the vertical axis of the small intestine. The duodenal enteroid model showed that electrogenic Na(+)/HCO3(-) cotransporter 1 might be a target in the intestinal mucosa for treatment of secretory diarrheas.
Keywords: Intestinal Organoids; NHE3 Activity; Na(+) Absorption; Stimulated Secretion.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures: The authors declare no financial or other interests with respect to this paper.
Figures




















References
-
- Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. - PubMed
-
- Sato T, Stange DE, Ferrante M, et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology. 2011;141:1762–1772. - PubMed
-
- Middendorp S, Schneeberger K, Wiegerinck CL, et al. Adult stem cells in the small intestine are intrinsically programmed with their location-specific function. Stem Cells. 2014;32:1083–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R24 DK099803/DK/NIDDK NIH HHS/United States
- R01-DK061765/DK/NIDDK NIH HHS/United States
- UH3-TR000504/TR/NCATS NIH HHS/United States
- UH3 TR000504/TR/NCATS NIH HHS/United States
- U19 AI116497/AI/NIAID NIH HHS/United States
- R01 DK026523/DK/NIDDK NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- T32 DK007632/DK/NIDDK NIH HHS/United States
- UH3-TR000503/TR/NCATS NIH HHS/United States
- P01 DK072084/DK/NIDDK NIH HHS/United States
- P01-DK072084/DK/NIDDK NIH HHS/United States
- K01-DK088950/DK/NIDDK NIH HHS/United States
- R01-DK026523/DK/NIDDK NIH HHS/United States
- P30 DK089502/DK/NIDDK NIH HHS/United States
- R01 DK061765/DK/NIDDK NIH HHS/United States
- P30-DK089502/DK/NIDDK NIH HHS/United States
- R01 AI080656/AI/NIAID NIH HHS/United States
- U18 TR000552/TR/NCATS NIH HHS/United States
- U18-TR000552/TR/NCATS NIH HHS/United States
- P30-DK056338/DK/NIDDK NIH HHS/United States
- T32-DK007632/DK/NIDDK NIH HHS/United States
- UH3 TR000503/TR/NCATS NIH HHS/United States
- P01 AI057788/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

