Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions
- PMID: 26677984
- PMCID: PMC4808451
- DOI: 10.1053/j.gastro.2015.11.049
Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions
Abstract
Background & aims: Gastric cancer develops in the context of parietal cell loss, spasmolytic polypeptide-expressing metaplasia (SPEM), and intestinal metaplasia (IM). We investigated whether expression of the activated form of Ras in gastric chief cells of mice leads to the development of SPEM, as well as progression of metaplasia.
Methods: We studied Mist1-CreERT2Tg/+;LSL-K-Ras(G12D)Tg/+ (Mist1-Kras) mice, which express the active form of Kras in chief cells on tamoxifen exposure. We studied Mist1-CreERT2Tg/+;LSL-KRas (G12D)Tg/+;R26RmTmG/+ (Mist1-Kras-mTmG) mice to examine whether chief cells that express active Kras give rise to SPEM and IM. Some mice received intraperitoneal injections of the Mitogen-activated protein kinase kinase (MEK) inhibitor, selumetinib, for 14 consecutive days. Gastric tissues were collected and analyzed by immunohistochemistry, immunofluorescence, and quantitative polymerase chain reaction.
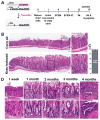
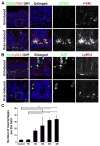
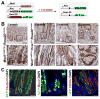
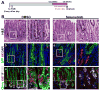
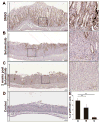
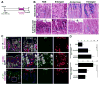
Results: Mist1-Kras mice developed metaplastic glands, which completely replaced normal fundic lineages and progressed to IM within 3-4 months after tamoxifen injection. The metaplastic glands expressed markers of SPEM and IM, and were infiltrated by macrophages. Lineage tracing studies confirmed that the metaplasia developed directly from Kras (G12D)-induced chief cells. Selumetinib induced persistent regression of SPEM and IM, and re-established normal mucosal cells, which were derived from normal gastric progenitor cells.
Conclusions: Expression of activated Ras in chief cells of Mist1-Kras mice led to the full range of metaplastic lineage transitions, including SPEM and IM. Inhibition of Ras signaling by inhibition of MEK might reverse preneoplastic metaplasia in the stomach.
Keywords: Carcinogenesis; Differentiation; MAP Kinase; Signal Transduction.
Copyright © 2016 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
-
- Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–66. - PubMed
-
- Goldenring JR, Nomura S. Differentiation of the Gastric Mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–G1004. - PubMed
-
- Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology. 2007;133:659–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

