How Dynein Moves Along Microtubules
- PMID: 26678005
- PMCID: PMC4706479
- DOI: 10.1016/j.tibs.2015.11.004
How Dynein Moves Along Microtubules
Abstract
Cytoplasmic dynein, a member of the AAA (ATPases Associated with diverse cellular Activities) family of proteins, drives the processive movement of numerous intracellular cargos towards the minus end of microtubules. Here, we summarize the structural and motile properties of dynein and highlight features that distinguish this motor from kinesin-1 and myosin V, two well-studied transport motors. Integrating information from recent crystal and cryoelectron microscopy structures, as well as high-resolution single-molecule studies, we also discuss models for how dynein biases its movement in one direction along a microtubule track, and present a movie that illustrates these principles.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
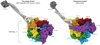
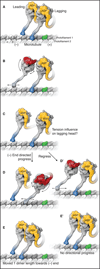
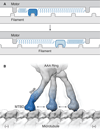
References
-
- Allan VJ. Cytoplasmic dynein. Biochem. Soc. Trans. 2011;39:1169–1178. - PubMed
-
- Verhey KJ, et al. Kinesin assembly and movement in cells. Annu. Rev. Biophys. 2011;40:267–288. - PubMed
-
- Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. - PubMed
-
- Hammer JA, Sellers JR. Walking to work: roles for class V myosins as cargo transporters. Nat. Rev. Mol. Cell Biol. 2012;13:13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

