Mechanism for attenuated outward conductance induced by mutations in the cytoplasmic pore of Kir2.1 channels
- PMID: 26678093
- PMCID: PMC4683409
- DOI: 10.1038/srep18404
Mechanism for attenuated outward conductance induced by mutations in the cytoplasmic pore of Kir2.1 channels
Abstract
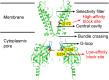
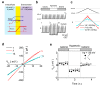
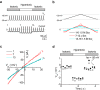
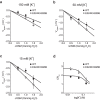
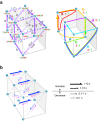
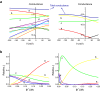
Outward currents through Kir2.1 channels regulate the electrical properties of excitable cells. These currents are subject to voltage-dependent attenuation by the binding of polyamines to high- and low-affinity sites, which leads to inward rectification, thereby controlling cell excitability. To examine the effects of positive charges at the low-affinity site in the cytoplasmic pore on inward rectification, we studied a mutant Kir channel (E224K/H226E) and measured single-channel currents and streaming potentials (Vstream), the latter provide the ratio of water to ions queued in a single-file permeation process in the selectivity filter. The water-ion coupling ratio was near one at a high K(+) concentration ([K(+)]) for the wild-type channel and increased substantially as [K(+)] decreased. On the other hand, fewer ions occupied the selectivity filter in the mutant at all [K(+)]. A model for the Kir channel involving a K(+) binding site in the wide pore was introduced. Model analyses revealed that the rate constants associated with the binding and release to and from the wide-pore K(+) binding site was modified in the mutant. These effects lead to the reduced contribution of a conventional two-ion permeation mode to total conductance, especially at positive potentials, thereby inward rectification.
Figures

Similar articles
-
Functional roles of charged amino acid residues on the wall of the cytoplasmic pore of Kir2.1.J Gen Physiol. 2006 Apr;127(4):401-19. doi: 10.1085/jgp.200509434. Epub 2006 Mar 13. J Gen Physiol. 2006. PMID: 16533896 Free PMC article.
-
Electrostatics in the cytoplasmic pore produce intrinsic inward rectification in kir2.1 channels.J Gen Physiol. 2005 Dec;126(6):551-62. doi: 10.1085/jgp.200509367. J Gen Physiol. 2005. PMID: 16316974 Free PMC article.
-
Voltage-dependent inhibition of outward Kir2.1 currents by extracellular spermine.Biochim Biophys Acta. 2013 Feb;1828(2):765-75. doi: 10.1016/j.bbamem.2012.08.016. Epub 2012 Aug 29. Biochim Biophys Acta. 2013. PMID: 22948070
-
Ser165 in the second transmembrane region of the Kir2.1 channel determines its susceptibility to blockade by intracellular Mg2+.J Gen Physiol. 2002 Nov;120(5):677-93. doi: 10.1085/jgp.20028663. J Gen Physiol. 2002. PMID: 12407079 Free PMC article.
-
Nutrigenomics of inward rectifier potassium channels.Biochim Biophys Acta Mol Basis Dis. 2023 Oct;1869(7):166803. doi: 10.1016/j.bbadis.2023.166803. Epub 2023 Jul 3. Biochim Biophys Acta Mol Basis Dis. 2023. PMID: 37406972 Review.
Cited by
-
Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory.Proc Natl Acad Sci U S A. 2021 Mar 23;118(12):e2017168118. doi: 10.1073/pnas.2017168118. Proc Natl Acad Sci U S A. 2021. PMID: 33741736 Free PMC article.
-
Origin of the Shape of Current-Voltage Curve through Nanopores: A Molecular Dynamics Study.Sci Rep. 2016 May 11;6:25750. doi: 10.1038/srep25750. Sci Rep. 2016. PMID: 27167118 Free PMC article.
-
An Unorthodox Mechanism Underlying Voltage Sensitivity of TRPV1 Ion Channel.Adv Sci (Weinh). 2020 Sep 21;7(20):2000575. doi: 10.1002/advs.202000575. eCollection 2020 Oct. Adv Sci (Weinh). 2020. PMID: 33101845 Free PMC article.
-
Queueing arrival and release mechanism for K+ permeation through a potassium channel.J Physiol Sci. 2019 Nov;69(6):919-930. doi: 10.1007/s12576-019-00706-4. Epub 2019 Aug 27. J Physiol Sci. 2019. PMID: 31456113 Free PMC article.
References
-
- Noble D. in The initiation of the heart beat. (Oxford University Press, 1979).
-
- Dhamoon A. S. et al. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res 94, 1332–1339 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

