Gasotransmitters in cancer: from pathophysiology to experimental therapy
- PMID: 26678620
- PMCID: PMC5319818
- DOI: 10.1038/nrd.2015.1
Gasotransmitters in cancer: from pathophysiology to experimental therapy
Abstract
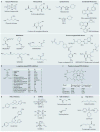
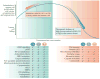
The three endogenous gaseous transmitters - nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) - regulate a number of key biological functions. Emerging data have revealed several new mechanisms for each of these three gasotransmitters in tumour biology. It is now appreciated that they show bimodal pharmacological character in cancer, in that not only the inhibition of their biosynthesis but also elevation of their concentration beyond a certain threshold can exert anticancer effects. This Review discusses the role of each gasotransmitter in cancer and the effects of pharmacological agents - some of which are in early-stage clinical studies - that modulate the levels of each gasotransmitter. A clearer understanding of the pharmacological character of these three gases and the mechanisms underlying their biological effects is expected to guide further clinical translation.
Conflict of interest statement
Competing interests
The author declares competing interests: see Web version for details.
Figures

References
-
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. - PubMed
-
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. - PubMed
-
- Liaudet L, Soriano FG, Szabo C. Biology of nitric oxide signaling. Crit Care Med. 2000;28:N37–52. - PubMed
-
- Ignarro LJ, editor. Nitric Oxide, Biology and Pathobiology. 2. Academic Press; 2009.
-
- Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

