A local-optimization refinement algorithm in single particle analysis for macromolecular complex with multiple rigid modules
- PMID: 26678751
- PMCID: PMC4707152
- DOI: 10.1007/s13238-015-0229-2
A local-optimization refinement algorithm in single particle analysis for macromolecular complex with multiple rigid modules
Abstract
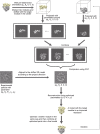
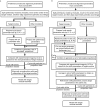
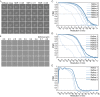
Single particle analysis, which can be regarded as an average of signals from thousands or even millions of particle projections, is an efficient method to study the three-dimensional structures of biological macromolecules. An intrinsic assumption in single particle analysis is that all the analyzed particles must have identical composition and conformation. Thus specimen heterogeneity in either composition or conformation has raised great challenges for high-resolution analysis. For particles with multiple conformations, inaccurate alignments and orientation parameters will yield an averaged map with diminished resolution and smeared density. Besides extensive classification approaches, here based on the assumption that the macromolecular complex is made up of multiple rigid modules whose relative orientations and positions are in slight fluctuation around equilibriums, we propose a new method called as local optimization refinement to address this conformational heterogeneity for an improved resolution. The key idea is to optimize the orientation and shift parameters of each rigid module and then reconstruct their three-dimensional structures individually. Using simulated data of 80S/70S ribosomes with relative fluctuations between the large (60S/50S) and the small (40S/30S) subunits, we tested this algorithm and found that the resolutions of both subunits are significantly improved. Our method provides a proof-of-principle solution for high-resolution single particle analysis of macromolecular complexes with dynamic conformations.
Keywords: conformational heterogeneity; cryo-electron microscopy; local optimization refinement; rigid module; single particle analysis.
Figures




References
-
- Bangliang S, Yiheng Z, Lihui P, Danya Y, Baofen Z. The use of simultaneous iterative reconstruction technique for electrical capacitance tomography. Chem Eng J. 2000;77:37–41. doi: 10.1016/S1385-8947(99)00134-5. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

