Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy
- PMID: 26678809
- PMCID: PMC4753054
- DOI: 10.1038/nrendo.2015.216
Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy
Abstract
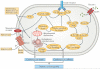
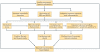
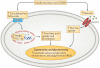
Insulin resistance, type 2 diabetes mellitus and associated hyperinsulinaemia can promote the development of a specific form of cardiomyopathy that is independent of coronary artery disease and hypertension. Termed diabetic cardiomyopathy, this form of cardiomyopathy is a major cause of morbidity and mortality in developed nations, and the prevalence of this condition is rising in parallel with increases in the incidence of obesity and type 2 diabetes mellitus. Of note, female patients seem to be particularly susceptible to the development of this complication of metabolic disease. The diabetic cardiomyopathy observed in insulin- resistant or hyperinsulinaemic states is characterized by impaired myocardial insulin signalling, mitochondrial dysfunction, endoplasmic reticulum stress, impaired calcium homeostasis, abnormal coronary microcirculation, activation of the sympathetic nervous system, activation of the renin-angiotensin-aldosterone system and maladaptive immune responses. These pathophysiological changes result in oxidative stress, fibrosis, hypertrophy, cardiac diastolic dysfunction and eventually systolic heart failure. This Review highlights a surge in diabetic cardiomyopathy research, summarizes current understanding of the molecular mechanisms underpinning this condition and explores potential preventive and therapeutic strategies.
Figures
References
-
- Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014;19:15–23. - PubMed
-
- Dhalla NS, Takeda N, Rodriguez-Leyva D, Elimban V. Mechanisms of subcellular remodeling in heart failure due to diabetes. Heart Fail. Rev. 2014;19:87–99. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

