Effects of storage on mixed-culture biological electrodes
- PMID: 26678949
- PMCID: PMC4683449
- DOI: 10.1038/srep18433
Effects of storage on mixed-culture biological electrodes
Abstract
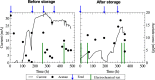
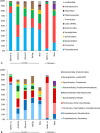
Storage methods are important to preserve the viability and biochemical characteristics of microbial cultures between experiments or during periods when bioreactors are inactive. Most of the research on storage has focused on isolates; however, there is an increasing interest in methods for mixed cultures, which are of relevance in environmental biotechnology. The purpose of this study was to investigate the effect of different storage methods on electrochemically active enrichment cultures. Acetate-oxidizing bioanodes generating a current density of about 5 A m(-2) were enriched in a microbial electrolysis cell. The effect of five weeks of storage was evaluated using electrochemical techniques and microbial community analysis. Storage by refrigeration resulted in quicker re-activation than freezing in 10% glycerol, while the bioelectrochemical activity was entirely lost after storage using dehydration. The results showed that the bioelectrochemical activity of bioanodes stored at low temperature could be retained. However, during the re-activation period the bioanodes only recovered 75% of the current density generated before storage and the bacterial communities were different in composition and more diverse after storage than before.
Figures



References
-
- Bae B.-U., Shin H.-S., Paik B.-C. & Chung J.-C. Re-activation characteristics of preserved anaerobic granular sludge. Bioresource Technology 53, 231–235 (1995).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

