Polyoxometalate-based homochiral metal-organic frameworks for tandem asymmetric transformation of cyclic carbonates from olefins
- PMID: 26678963
- PMCID: PMC4703842
- DOI: 10.1038/ncomms10007
Polyoxometalate-based homochiral metal-organic frameworks for tandem asymmetric transformation of cyclic carbonates from olefins
Abstract
Currently, great interest is focused on developing auto-tandem catalytic reactions; a substrate is catalytically transferred through mechanistically distinct reactions without altering any reaction conditions. Here by incorporating a pyrrolidine moiety as a chiral organocatalyst and a polyoxometalate as an oxidation catalyst, a powerful approach is devised to achieve a tandem catalyst for the efficient conversion of CO2 into value-added enantiomerically pure cyclic carbonates. The multi-catalytic sites are orderly distributed and spatially matched in the framework. The captured CO2 molecules are synergistically fixed and activated by well-positioned pyrrolidine and amine groups, providing further compatibility with the terminal W=O activated epoxidation intermediate and driving the tandem catalytic process in a single workup stage and an asymmetric fashion. The structural simplicity of the building blocks and the use of inexpensive and readily available chemical reagents render this approach highly promising for the development of practical homochiral materials for CO2 conversion.
Figures

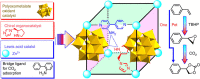
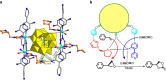
References
-
- Raman S. K., Brulé E., Tschan M. J. L. & Thomas C. M. Tandem catalysis: a new approach to polypeptides and cyclic carbonates. Chem. Commun. 50, 13773–13776 (2014). - PubMed
-
- Zhu Y. G., Wang Q., Cornwall R. G. & Shi Y. A. Metal-catalyzed asymmetric sulfoxidation, epoxidation and hydroxylation by hydrogen peroxide. Chem. Rev. 114, 8199–8256 (2014). - PubMed
-
- Katsuki T. & Sharpless K. B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 102, 5974–5976 (1980).
-
- Zhang S. L., Huang Y. Z., Jing H. W., Yao W. X. & Yan P. Chiral ionic liquids improved the asymmetric cycloaddition of CO2 to epoxides. Green Chem. 11, 935–938 (2009).
-
- Ren W. M., Liu Y. & Lu X. B. Bifunctional Aluminum Catalyst for CO2 Fixation: Regioselective ring opening of three-membered heterocyclic compounds. J. Org. Chem. 79, 9771–9777 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

