Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet
- PMID: 26679056
- PMCID: PMC4828297
- DOI: 10.1002/mnfr.201500814
Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFκB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet
Abstract
Scope: Green tea extract (GTE) reduces liver steatosis and inflammation during nonalcoholic steatohepatitis (NASH). We hypothesized GTE would mitigate NASH in a nuclear factor erythroid-2-related-factor-2 (Nrf2)-dependent manner in a high fat (HF) induced model.
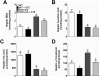
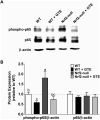
Methods and results: Nrf2-null and wild-type (WT) mice were fed an HF diet containing 0 or 2% GTE for eight weeks prior to assessing parameters of NASH. Compared to WT mice, Nrf2-null mice had increased serum alanine aminotransferase, hepatic triglyceride, expression of free fatty acid uptake and lipogenic genes, malondialdehyde and NFκB phosphorylation and expression of pro-inflammatory genes. In WT mice, GTE increased Nrf2 and NADPH:quinone oxidoreductase-1 mRNA, and lowered hepatic steatosis, lipid uptake and lipogenic gene expression, malondialdehyde, and NFκB-dependent inflammation. In Nrf2-null mice, GTE lowered NFκB phosphorylation and TNF-α and MCP1 mRNA to levels observed in WT mice fed GTE whereas hepatic triglyceride and lipogenic genes were lowered only to those of WT mice fed no GTE. Malondialdehyde was lowered in Nrf2-null mice fed GTE, but not to levels of WT mice, and without improving the hepatic antioxidants α-tocopherol, ascorbic acid and uric acid.
Conclusion: Nrf2 deficiency exacerbates NASH whereas anti-inflammatory and hypolipidemic activities of GTE likely occur largely independent of Nrf2 signaling.
Keywords: Green tea; Inflammation; NASH; Nrf2; Oxidative stress.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

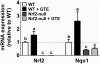


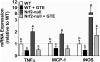
Similar articles
-
Green tea extract treatment reduces NFκB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability.J Nutr Biochem. 2017 Mar;41:34-41. doi: 10.1016/j.jnutbio.2016.12.007. Epub 2016 Dec 21. J Nutr Biochem. 2017. PMID: 28038359
-
Green Tea Lowers Hepatic COX-2 and Prostaglandin E2 in Rats with Dietary Fat-Induced Nonalcoholic Steatohepatitis.J Med Food. 2015 Jun;18(6):648-55. doi: 10.1089/jmf.2014.0048. Epub 2014 Dec 2. J Med Food. 2015. PMID: 25453513
-
Green tea extract protects against hepatic NFκB activation along the gut-liver axis in diet-induced obese mice with nonalcoholic steatohepatitis by reducing endotoxin and TLR4/MyD88 signaling.J Nutr Biochem. 2018 Mar;53:58-65. doi: 10.1016/j.jnutbio.2017.10.016. Epub 2017 Nov 3. J Nutr Biochem. 2018. PMID: 29190550
-
Anti-inflammatory activities of green tea catechins along the gut-liver axis in nonalcoholic fatty liver disease: lessons learned from preclinical and human studies.J Nutr Biochem. 2020 Nov;85:108478. doi: 10.1016/j.jnutbio.2020.108478. Epub 2020 Aug 12. J Nutr Biochem. 2020. PMID: 32801031 Review.
-
The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism.Biomed Res Int. 2015;2015:597134. doi: 10.1155/2015/597134. Epub 2015 May 18. Biomed Res Int. 2015. PMID: 26120584 Free PMC article. Review.
Cited by
-
Green Tea Extract Treatment in Obese Mice with Nonalcoholic Steatohepatitis Restores the Hepatic Metabolome in Association with Limiting Endotoxemia-TLR4-NFκB-Mediated Inflammation.Mol Nutr Food Res. 2019 Dec;63(24):e1900811. doi: 10.1002/mnfr.201900811. Epub 2019 Oct 9. Mol Nutr Food Res. 2019. PMID: 31574193 Free PMC article.
-
High-Fat Diet Aggravates the Intestinal Barrier Injury via TLR4-RIP3 Pathway in a Rat Model of Severe Acute Pancreatitis.Mediators Inflamm. 2019 Dec 17;2019:2512687. doi: 10.1155/2019/2512687. eCollection 2019. Mediators Inflamm. 2019. PMID: 31933540 Free PMC article.
-
N-Acetylcysteine Reduced Ischemia and Reperfusion Damage Associated with Steatohepatitis in Mice.Int J Mol Sci. 2020 Jun 9;21(11):4106. doi: 10.3390/ijms21114106. Int J Mol Sci. 2020. PMID: 32526845 Free PMC article.
-
Combined effects of green tea supplementation and eccentric exercise on nuclear factor erythroid 2-related factor 2 activity.Eur J Appl Physiol. 2024 Jan;124(1):245-256. doi: 10.1007/s00421-023-05271-8. Epub 2023 Jul 13. Eur J Appl Physiol. 2024. PMID: 37439906 Free PMC article.
-
CCM111 prevents hepatic fibrosis via cooperative inhibition of TGF-β, Wnt and STAT3 signaling pathways.J Food Drug Anal. 2019 Jan;27(1):184-194. doi: 10.1016/j.jfda.2018.09.008. Epub 2018 Oct 25. J Food Drug Anal. 2019. PMID: 30648571 Free PMC article.
References
-
- Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Dig Dis Sci. 2005;50:171–180. - PubMed
-
- Wong VW, Wong GL, Choi PC, Chan AW, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. - PubMed
-
- Angulo P. Obesity and nonalcoholic fatty liver disease. Nutr Rev. 2007;65:S57–63. - PubMed
-
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. - PubMed
-
- Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–678. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

