Abasic pivot substitution harnesses target specificity of RNA interference
- PMID: 26679372
- PMCID: PMC4703836
- DOI: 10.1038/ncomms10154
Abasic pivot substitution harnesses target specificity of RNA interference
Abstract
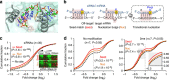
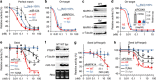
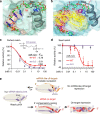
Gene silencing via RNA interference inadvertently represses hundreds of off-target transcripts. Because small interfering RNAs (siRNAs) can function as microRNAs, avoiding miRNA-like off-target repression is a major challenge. Functional miRNA-target interactions are known to pre-require transitional nucleation, base pairs from position 2 to the pivot (position 6). Here, by substituting nucleotide in pivot with abasic spacers, which prevent base pairing and alleviate steric hindrance, we eliminate miRNA-like off-target repression while preserving on-target activity at ∼ 80-100%. Specifically, miR-124 containing dSpacer pivot substitution (6pi) loses seed-mediated transcriptome-wide target interactions, repression activity and biological function, whereas other conventional modifications are ineffective. Application of 6pi allows PCSK9 siRNA to efficiently lower plasma cholesterol concentration in vivo, and abolish potentially deleterious off-target phenotypes. The smallest spacer, C3, also shows the same improvement in target specificity. Abasic pivot substitution serves as a general means to harness the specificity of siRNA experiments and therapeutic applications.
Conflict of interest statement
E.-S.J. and S.W.C. are inventors on a patent application describing the use of abasic pivot substitution as eliminating miRNA-like off-target effect of siRNAs. The remaining authors declare no competing financial interests.
Figures





References
-
- Hannon G. J. & Rossi J. J. Unlocking the potential of the human genome with RNA interference. Nature 431, 371–378 (2004). - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature 431, 350–355 (2004). - PubMed
-
- He L. & Hannon G. J. MicroRNAs: small RNAs with a big role in gene regulation. Nature Rev. Genet. 5, 522–531 (2004). - PubMed
-
- McManus M. T. & Sharp P. A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 3, 737–747 (2002). - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

