Demographic and Clinical Profiles of Type 2 Diabetes Mellitus Patients Initiating Canagliflozin Versus DPP-4 Inhibitors in a Large U.S. Managed Care Population
- PMID: 26679969
- PMCID: PMC10398281
- DOI: 10.18553/jmcp.2015.21.12.1204
Demographic and Clinical Profiles of Type 2 Diabetes Mellitus Patients Initiating Canagliflozin Versus DPP-4 Inhibitors in a Large U.S. Managed Care Population
Abstract
Background: Canagliflozin is the first sodium-glucose co-transporter-2 (SGLT-2) inhibitor-a new class of oral antidiabetic (OAD) medication-approved for type 2 diabetes mellitus (T2DM) treatment in the United States. Approved less than 2 years ago, use of canagliflozin is largely uncharacterized.
Objective: To investigate and compare baseline demographic, clinical, and economic characteristics of patients initiating canagliflozin and dipeptidyl peptidase-4 (DPP-4) inhibitors in the real-world setting.
Methods: Using administrative claims data from a large, geographically diverse U.S. managed care organization, this retrospective study assessed adult T2DM patients (aged ≥ 18 years) initiating treatment with canagli-flozin or DPP-4 agents. Eligible patients had ≥1 medical claim with a T2DM diagnosis and ≥ 1 outpatient pharmacy claim for canagliflozin or a DPP-4 agent between January 1, 2011, and September 30, 2013. Patients with ≥ 1 canagliflozin fill were selected first and assigned to the canagliflozin cohort following a hierarchical approach; the date of the earliest canagliflozin fill was defined as the index date. Remaining patients with DPP-4 fills were then assigned to the DPP-4 cohort, with the index date as the first DPP-4 fill. Only patients with at least 12 months of pre-index (baseline) enrollment were included. Patients with fills for their cohort-defining drug over 3 months before the index date were excluded in order to focus on new initiators. A subset of patients with ≥ 3 months of continuous enrollment following their index dates was used to examine medication patterns after initiation. Patients with hyperglycemia; type 1, gestational, or nonclinical diabetes; or diabetes with hyperosmolar coma were excluded. Demographic, clinical, and economic characteristics were assessed over baseline and compared using two-sample t-tests or chi-square/Fisher's exact tests. Multivariable logistic regression models were built to assess baseline factors associated with initiation of canagliflozin versus DPP-4.
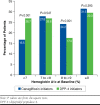
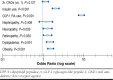
Results: Overall, 1,566 patients initiated canagliflozin, and 26,224 patients initiated DPP-4 treatment. Males constituted slightly more than 60% of each treatment group; mean age was approximately 55 years in each cohort. A significantly smaller proportion of canagliflozin patients (41.3%) initiated treatment with endocrinologists compared with DPP-4 patients (69.2%, P less than 0.001), and canagliflozin patients were more likely (29.4%) to initiate treatment with a primary care physician compared with DPP-4 patients (9.9%, P less than 0.001). Comorbidities were present more frequently in canagliflozin initiators: nephropathy (10.6% vs. 7.0%), retinopathy (10.4% vs. 7.5%), dyslipidemia (82.4% vs. 72.2%), and obesity (24.9% vs. 15.6%), respectively (P less than 0.001 for all comparisons). The mean (SD) Quan-Charlson Comorbidity Index score was greater for canagliflozin, 1.05 (1.7), compared with DPP-4 initiators, 0.92 (1.6), P = 0.002. Among the subset of patients with available hemoglobin A1c (A1c) results, a significantly smaller proportion of canagliflozin initiators (16.5%) versus DPP-4 initiators (26.7%) were at the A1c less than 7% treatment goal at baseline (P less than 0.001). Among patients with 3 months follow-up, 89.2% of canagliflozin and 75.1% of DPP-4 initiators had ≥ 1 fill for their index drugs over this time frame. Canagliflozin initiators had significantly greater baseline utilization of office visits, endocrinologist and outpatient services, and more prescription fills. Total diabetes-related medical costs at baseline ($3,025 vs. $3,477 for canagliflozin and DPP-4 initiators) were not significantly different, while mean diabetes-related pharmacy costs were higher in the canagliflozin group ($4,037 vs. $1,411, P less than 0.001). Regression analysis indicated that baseline insulin and glucagon-like peptide-1 use, as well as comorbid dyslipidemia and obesity, were significantly associated with the initiation of canagliflozin versus DPP-4 agents.
Conclusions: In this sample of commercially insured patients within a large managed care plan, canagliflozin was often initiated as second- or third-line therapy, with a relatively high share of patients receiving concomitant antidiabetic injectables, compared with DPP-4 initiators. Canagliflozin initiators had highly elevated A1c levels and were frequently diagnosed with other metabolic conditions. Baseline pharmacy utilization and costs were higher among canagliflozin patients. Future research is needed to assess real-world clinical outcomes after canagliflozin initiation, while taking these baseline differences into account.
Conflict of interest statement
This study was funded by Eli Lilly and Company. Grabner and Geremakis are employees of HealthCore, an independent research organization that received funding from Eli Lilly and Company for the conduct of the study. Peng and Bae are employees of Eli Lilly and Company.
All authors contributed equally to the study design, interpretation of data, and writing and revision of the manuscript. Grabner and Geremakis collected the data.
Figures
References
-
- Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed October 22, 2015.
-
- Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-.... Accessed November 3, 2015.
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR.. Prevalence and trends in obesity among U.S. adults, 1999-2008. JAMA. 2010;303(3):235-41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous