Deformability of Tumor Cells versus Blood Cells
- PMID: 26679988
- PMCID: PMC4683468
- DOI: 10.1038/srep18542
Deformability of Tumor Cells versus Blood Cells
Abstract
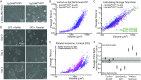
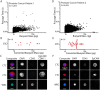
The potential for circulating tumor cells (CTCs) to elucidate the process of cancer metastasis and inform clinical decision-making has made their isolation of great importance. However, CTCs are rare in the blood, and universal properties with which to identify them remain elusive. As technological advancements have made single-cell deformability measurements increasingly routine, the assessment of physical distinctions between tumor cells and blood cells may provide insight into the feasibility of deformability-based methods for identifying CTCs in patient blood. To this end, we present an initial study assessing deformability differences between tumor cells and blood cells, indicated by the length of time required for them to pass through a microfluidic constriction. Here, we demonstrate that deformability changes in tumor cells that have undergone phenotypic shifts are small compared to differences between tumor cell lines and blood cells. Additionally, in a syngeneic mouse tumor model, cells that are able to exit a tumor and enter circulation are not required to be more deformable than the cells that were first injected into the mouse. However, a limited study of metastatic prostate cancer patients provides evidence that some CTCs may be more mechanically similar to blood cells than to typical tumor cell lines.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Allard W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–904 (2004). - PubMed
-
- Alix-Panabieŕes C. & Pantel K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 59, 110–118 (2013). - PubMed
-
- Alunni-fabbroni M. & Teresa M. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods 50, 289–297 (2010). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

