Rabphilin 3A retains NMDA receptors at synaptic sites through interaction with GluN2A/PSD-95 complex
- PMID: 26679993
- PMCID: PMC4703873
- DOI: 10.1038/ncomms10181
Rabphilin 3A retains NMDA receptors at synaptic sites through interaction with GluN2A/PSD-95 complex
Abstract
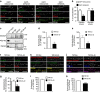
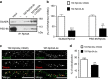
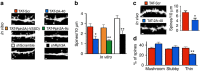
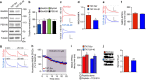
NMDA receptor (NMDAR) composition and synaptic retention represent pivotal features in the physiology and pathology of excitatory synapses. Here, we identify Rabphilin 3A (Rph3A) as a new GluN2A subunit-binding partner. Rph3A is known as a synaptic vesicle-associated protein involved in the regulation of exo- and endocytosis processes at presynaptic sites. We find that Rph3A is enriched at dendritic spines. Protein-protein interaction assays reveals that Rph3A N-terminal domain interacts with GluN2A(1349-1389) as well as with PSD-95(PDZ3) domains, creating a ternary complex. Rph3A silencing in neurons reduces the surface localization of synaptic GluN2A and NMDAR currents. Moreover, perturbing GluN2A/Rph3A interaction with interfering peptides in organotypic slices or in vivo induces a decrease of the amplitude of NMDAR-mediated currents and GluN2A density at dendritic spines. In conclusion, Rph3A interacts with GluN2A and PSD-95 forming a complex that regulates NMDARs stabilization at postsynaptic membranes.
Figures





Similar articles
-
Rabphilin 3A: A novel target for the treatment of levodopa-induced dyskinesias.Neurobiol Dis. 2017 Dec;108:54-64. doi: 10.1016/j.nbd.2017.08.001. Epub 2017 Aug 18. Neurobiol Dis. 2017. PMID: 28823933
-
Arg209Lys and Gln508His missense variants in Rabphilin 3A cause pre- and post-synaptic dysfunctions at excitatory glutamatergic synapses.Sci Rep. 2025 Mar 13;15(1):8698. doi: 10.1038/s41598-025-93403-9. Sci Rep. 2025. PMID: 40082528 Free PMC article.
-
Rabphilin-3A undergoes phase separation to regulate GluN2A mobility and surface clustering.Nat Commun. 2023 Jan 24;14(1):379. doi: 10.1038/s41467-023-36046-6. Nat Commun. 2023. PMID: 36693856 Free PMC article.
-
Protein-protein interactions at the NMDA receptor complex: From synaptic retention to synaptonuclear protein messengers.Neuropharmacology. 2021 Jun 1;190:108551. doi: 10.1016/j.neuropharm.2021.108551. Epub 2021 Apr 2. Neuropharmacology. 2021. PMID: 33819458 Review.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
-
Role of Rph3A in brain injury induced by experimental cerebral ischemia-reperfusion model in rats.CNS Neurosci Ther. 2022 Jul;28(7):1124-1138. doi: 10.1111/cns.13850. Epub 2022 Apr 25. CNS Neurosci Ther. 2022. PMID: 35467084 Free PMC article.
-
DDX60 Is Associated With Glioma Malignancy and Serves as a Potential Immunotherapy Biomarker.Front Oncol. 2021 Jun 10;11:665360. doi: 10.3389/fonc.2021.665360. eCollection 2021. Front Oncol. 2021. PMID: 34178649 Free PMC article.
-
In vivo Roles of Rab27 and Its Effectors in Exocytosis.Cell Struct Funct. 2021 Nov 6;46(2):79-94. doi: 10.1247/csf.21043. Epub 2021 Sep 4. Cell Struct Funct. 2021. PMID: 34483204 Free PMC article. Review.
-
TIA1 is a gender-specific disease modifier of a mild mouse model of spinal muscular atrophy.Sci Rep. 2017 Aug 3;7(1):7183. doi: 10.1038/s41598-017-07468-2. Sci Rep. 2017. PMID: 28775379 Free PMC article.
-
Visualizing the triheteromeric N-methyl-D-aspartate receptor subunit composition.Front Synaptic Neurosci. 2023 May 24;15:1156777. doi: 10.3389/fnsyn.2023.1156777. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 37292368 Free PMC article.
References
-
- Papouin T. et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

