Long-term outcomes of neovascular glaucoma treated with and without intravitreal bevacizumab
- PMID: 26681145
- PMCID: PMC4791706
- DOI: 10.1038/eye.2015.259
Long-term outcomes of neovascular glaucoma treated with and without intravitreal bevacizumab
Abstract
Aims: To compare the outcomes of neovascular glaucoma (NVG) treated with and without intravitreal bevacizumab in a large case comparison study.
Methods: The study is a retrospective, comparative, case series of 163 eyes of 151 patients with NVG, including 99 treated without and 64 treated with intravitreal bevacizumab. Medical and surgical treatments for NVG were assessed. The main outcome measures were visual acuity (VA) and intraocular pressure (IOP).
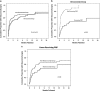
Results: At the time of NVG diagnosis, the median VA was count fingers (CF) in the non-bevacizumab group and 2/300 in the bevacizumab group. IOP (mean±SD) was 43.1±13.0 mm Hg in the non-bevacizumab group and 40.8±11.5 mm Hg in the bevacizumab group. IOP (mean±SD) decreased to 18.3±13.8 mm Hg in the non-bevacizumab group and 15.3±8.0 mm Hg in the bevacizumab group, and the median VA was CF in both treatment groups at a mean follow-up of 12 months. Panretinal photocoagulation (PRP) substantially reduced the need for glaucoma surgery (P<0.001) in bevacizumab treated NVG eyes.
Conclusions: Although bevacizumab delayed the need for glaucoma surgery, PRP was the most important factor that reduced the need for surgery. Vision and IOP in eyes with NVG treated with bevacizumab showed no long-term differences when compared with eyes that were not treated with bevacizumab. Thus, intravitreal bevacizumab serves as an effective temporizing treatment, but is not a replacement for close monitoring and definitive treatment of NVG. PRP remains the treatment modality that affects the course of NVG in terms of decreasing the need for surgery to control IOP.
Conflict of interest statement
PJR received research support from Acucela, Apellis, Genentech/Roche, GlaxoSmithKline, Neurotech, Ocata Therapeutics, and Tyrogenex. He is a consultant for Achillion, Acucela, Alcon, Bayer, Chengdu Kanghong Biotech, CoDa Therapeutics, Genentech/Roche, Healios K.K., Merck, Regeneron, Stealth and Tyrogenex. LCO is on the Scientific Advisory Board for: Alcon surgical, ScienceBased Health (none of them relevant to the published work). The other authors have no financial interests in any of the products discussed in this article. The Bascom Palmer Eye Institute is supported by NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant.
RKL, LCO, ALM, MSS, PJR and SJG participated in the conception and design of this study, analysis, and interpretation of data. WS and WJF participated in the statistical analysis and interpretation of the data. RKL, LCO, and MSS participated in drafting the article and revising it critically for important intellectual content and final approval of the version to be published. Ethics approval was provided by the University of Miami Miller School of Medicine Institutional Review Board.
Figures
Comment in
-
Reply to 'Comment on: Long-term outcomes of neovascular glaucoma treated with and without intravitreal bevacizumab'.Eye (Lond). 2016 Jun;30(6):897-8. doi: 10.1038/eye.2016.32. Epub 2016 Mar 4. Eye (Lond). 2016. PMID: 26939556 Free PMC article. No abstract available.
-
Comment on: 896.Eye (Lond). 2016 Jun;30(6):896-7. doi: 10.1038/eye.2016.31. Epub 2016 Mar 4. Eye (Lond). 2016. PMID: 26939563 Free PMC article. No abstract available.
References
-
- John T, Sassani JW, Eagle RC Jr. The myofibroblastic component of rubeosis iridis. Ophthalmology 1983; 90(6): 721–728. - PubMed
-
- Shazly TA. Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol 2009; 24(2): 113–121. - PubMed
-
- Gartner S, Henkind P. Neovascularization of the iris (rubeosis iridis). Surv Ophthalmol 1978; 22(5): 291–312. - PubMed
-
- Brown GC, Magargal LE, Schachat A, Shah H. Neovascular glaucoma. Etiologic considerations. Ophthalmology 1984; 91(4): 315–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

