Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research
- PMID: 26681259
- PMCID: PMC4769676
- DOI: 10.1111/jne.12351
Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research
Abstract
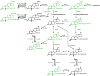
Neuroactive steroids are endogenous neuromodulators synthesised in the brain that rapidly alter neuronal excitability by binding to membrane receptors, in addition to the regulation of gene expression via intracellular steroid receptors. Neuroactive steroids induce potent anxiolytic, antidepressant, anticonvulsant, sedative, analgesic and amnesic effects, mainly through interaction with the GABAA receptor. They also exert neuroprotective, neurotrophic and antiapoptotic effects in several animal models of neurodegenerative diseases. Neuroactive steroids regulate many physiological functions, such as the stress response, puberty, the ovarian cycle, pregnancy and reward. Their levels are altered in several neuropsychiatric and neurological diseases and both preclinical and clinical studies emphasise a therapeutic potential of neuroactive steroids for these diseases, whereby symptomatology ameliorates upon restoration of neuroactive steroid concentrations. However, direct administration of neuroactive steroids has several challenges, including pharmacokinetics, low bioavailability, addiction potential, safety and tolerability, which limit its therapeutic use. Therefore, modulation of neurosteroidogenesis to restore the altered endogenous neuroactive steroid tone may represent a better therapeutic approach. This review summarises recent approaches that target the neuroactive steroid biosynthetic pathway at different levels aiming to promote neurosteroidogenesis. These include modulation of neurosteroidogenesis through ligands of the translocator protein 18 kDa and the pregnane xenobiotic receptor, as well as targeting of specific neurosteroidogenic enzymes such as 17β-hydroxysteroid dehydrogenase type 10 or P450 side chain cleavage. Enhanced neurosteroidogenesis through these targets may be beneficial not only for neurodegenerative diseases, such as Alzheimer's disease and age-related dementia, but also for neuropsychiatric diseases, including alcohol use disorders.
Keywords: 17β-hydroxysteroid dehydrogenase type 10; 3α,5α-THP; Alzheimer's disease; P450 side chain cleavage; alcoholism; neuroactive steroids; pregnane xenobiotic receptor; translocator protein 18 kDa.
© 2015 British Society for Neuroendocrinology.
Figures
References
-
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6(6):2311–22. - PubMed
-
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23(8):963–87. - PubMed
-
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6(7):565–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

