A test of the additivity of acute toxicity of binary-metal mixtures of ni with Cd, Cu, and Zn to Daphnia magna, using the inflection point of the concentration-response curves
- PMID: 26681657
- PMCID: PMC5764768
- DOI: 10.1002/etc.3342
A test of the additivity of acute toxicity of binary-metal mixtures of ni with Cd, Cu, and Zn to Daphnia magna, using the inflection point of the concentration-response curves
Abstract
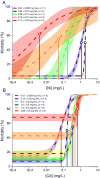
Mixtures of metals are often present in surface waters, leading to toxicity that is difficult to predict. To provide data for development of multimetal toxicity models, Daphnia magna neonates were exposed to individual metals (Cd, Cu, Ni, Zn) and to binary combinations of those metals in standard 48-h lethality tests conducted in US Environmental Protection Agency moderately hard reconstituted water with 3 mg dissolved organic carbon (DOC)/L added as Suwannee River fulvic acid. Toxicity tests were performed with mixtures of Ni and 1) Cd, which is considerably more toxic than Ni; 2) Cu, which is less toxic than Cd but more toxic than Ni; and 3) Zn, which has a toxicity threshold similar to Ni. For each combination of metals in the binary mixtures, the concentration of 1 metal was held constant while the second metal was varied through a series that ranged from nonlethal to lethal concentrations; then the roles of the metals were reversed. Inflection points of the concentration-response curves were compared to test for additivity of toxicity. Sublethal concentrations of Ni caused less-than-additive toxicity with Cd, slightly less-than-additive toxicity with Zn, and greater-than-additive toxicity with Cu. One explanation of these results might be competition among the metals for binding to biological ligands and/or dissolved organic matter. Therefore, models might have to incorporate sometimes competing chemical interactions to accurately predict metal-mixture toxicity. Environ Toxicol Chem 2016;35:1843-1851. © 2015 SETAC.
Keywords: Bioavailability; Independent action; Metal complexation; Metal-metal competition; Response addition.
© 2015 SETAC.
Figures



References
-
- Allen HE, Luther GW, Garrison W. Metals in Surface Waters. Ann Arbor, Chelsea MI, USA: 1997.
-
- Meyer JS, Farley KJ, Garman ER. Metal Mixtures Modeling Evaluation Project: 1. Background. Environ Toxicol Chem. 2015;34:726–740. - PubMed
-
- Bury N, Shaw J, Glover C, Hogstrand C. Derivation of a toxicity-based model to predict how water chemistry influences silver toxicity to invertebrates. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:259–270. - PubMed
-
- Meyer JS, Clearwater SJ, Doser TA, Rogaczewski MJ, Hansen JA. Effects of Water Chemistry on Bioavailability and Toxicity of Waterborne Cadmium, Copper, Nickel, Lead, and Zinc to Freshwater Organisms. SETAC; Pensacola, FL, USA: 2007.
-
- Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PG, Delos CG, Di Toro DM, Dwyer RL, Galvez F. The biotic ligand model: A historical overview. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133:3–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

