Intranasal Glucagon for Treatment of Insulin-Induced Hypoglycemia in Adults With Type 1 Diabetes: A Randomized Crossover Noninferiority Study
- PMID: 26681725
- PMCID: PMC4722945
- DOI: 10.2337/dc15-1498
Intranasal Glucagon for Treatment of Insulin-Induced Hypoglycemia in Adults With Type 1 Diabetes: A Randomized Crossover Noninferiority Study
Abstract
Objective: Treatment of severe hypoglycemia with loss of consciousness or seizure outside of the hospital setting is presently limited to intramuscular glucagon requiring reconstitution immediately prior to injection, a process prone to error or omission. A needle-free intranasal glucagon preparation was compared with intramuscular glucagon for treatment of insulin-induced hypoglycemia.
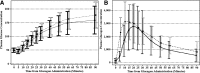
Research design and methods: At eight clinical centers, a randomized crossover noninferiority trial was conducted involving 75 adults with type 1 diabetes (mean age, 33 ± 12 years; median diabetes duration, 18 years) to compare intranasal (3 mg) versus intramuscular (1 mg) glucagon for treatment of hypoglycemia induced by intravenous insulin. Success was defined as an increase in plasma glucose to ≥70 mg/dL or ≥20 mg/dL from the glucose nadir within 30 min after receiving glucagon.
Results: Mean plasma glucose at time of glucagon administration was 48 ± 8 and 49 ± 8 mg/dL at the intranasal and intramuscular visits, respectively. Success criteria were met at all but one intranasal visit and at all intramuscular visits (98.7% vs. 100%; difference 1.3%, upper end of 1-sided 97.5% CI 4.0%). Mean time to success was 16 min for intranasal and 13 min for intramuscular (P < 0.001). Head/facial discomfort was reported during 25% of intranasal and 9% of intramuscular dosing visits; nausea (with or without vomiting) occurred with 35% and 38% of visits, respectively.
Conclusions: Intranasal glucagon was highly effective in treating insulin-induced hypoglycemia in adults with type 1 diabetes. Although the trial was conducted in a controlled setting, the results are applicable to real-world management of severe hypoglycemia, which occurs owing to excessive therapeutic insulin relative to the impaired or absent endogenous glucagon response.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

Comment in
-
Comment on Rickels et al. Intranasal Glucagon for Treatment of Insulin-Induced Hypoglycemia in Adults With Type 1 Diabetes: A Randomized Crossover Noninferiority Study. Diabetes Care 2016;39:264-270.Diabetes Care. 2016 Oct;39(10):e192. doi: 10.2337/dc16-0955. Diabetes Care. 2016. PMID: 27660134 No abstract available.
-
Response to Comment on Rickels et al. Intranasal Glucagon for Treatment of Insulin-Induced Hypoglycemia in Adults With Type 1 Diabetes: A Randomized Crossover Noninferiority Study. Diabetes Care 2016;39:264-270.Diabetes Care. 2016 Oct;39(10):e193-4. doi: 10.2337/dci16-0025. Diabetes Care. 2016. PMID: 27660135 Free PMC article. No abstract available.
References
-
- Brodows RG, Williams C, Amatruda JM. Treatment of insulin reactions in diabetics. JAMA 1984;252:3378–3381 - PubMed
-
- Slama G, Traynard PY, Desplanque N, et al. . The search for an optimized treatment of hypoglycemia. Carbohydrates in tablets, solutin, or gel for the correction of insulin reactions. Arch Intern Med 1990;150:589–593 - PubMed
-
- Gold AE, MacLeod KM, Deary IJ, Frier BM. Hypoglycemia-induced cognitive dysfunction in diabetes mellitus: effect of hypoglycemia unawareness. Physiol Behav 1995;58:501–511 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

